 Back
BackProblem 1
Which of the following is a Brønsted-Lowry base, but not an Arrhenius base? (LO 16.1) (a) HNO3 (b) CsOH (c) CH3NH2 (d) CH3OHProblem 2
The following pictures represent equal volumes of aqueous solutions of three acids HA (A = X, Y, or Z); water molecules have been omitted for clarity. Which is the strongest acid?
(a) HX (b) HY (c) HZ (d) All three acids are strong acids and have equal strength.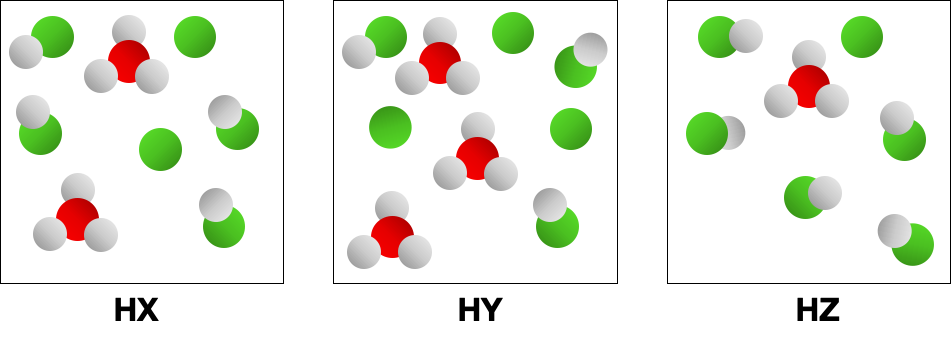
Problem 3
Consider the conjugate bases, 1X-, Y-, Z-2 in Problem 2. If you mix equal concentrations of reactants and products, which of the following reactions will proceed to the left? (LO 16.3) (a) HX + Y- HY + X- (b) HX + Z- HZ + X- (c) HY + X- HX + Y- (d) HZ + Y- HY + Z-
Problem 4
Which is the strongest acid? (LO 16.4) (a) HClO3 (b) HBrO3 (c) H2SO3 (d) H2TeO3Problem 5
What is the concentration of hydroxide ions 3OH-4 in a glass of wine with pH = 3.64? (LO 16.5, 16.6) (a) 2.3 * 10-4 M (b) 6.4 * 10-3 M (c) 6.8 * 10-9 M (d) 4.4 * 10-11 MProblem 6
What is the pH of an aqueous solution of Ca1OH22 at 25.0 °C with a concentration of 6.3 * 10-5 M? (LO 16.7) (a) 4.20 (b) 10.10 (c) 11.36 (d) 9.80Problem 7
An acid solution with a concentration of 0.500 M has a pH = 3.21. What is the Ka of the acid? (LO 16.8) (a) 1.2 * 10-5 (b) 1.7 * 10-6 (c) 7.6 * 10-7 (d) 5.4 * 10-3Problem 10
Ammonia 1NH32 has base dissociation constant 1Kb2 of 1.8 * 10-5. What is the concentration of an aqueous ammonia solution that has a pH of 11.68? (LO 16.11) (a) 0.28 M (b) 3.6 M (c) 9.0 * 10-3 M (d) 1.3 MProblem 13
Consider the reaction: SO2 + OH- S HSO3 -. Which reaction scheme shows the correct use of the curved arrow notation representing the donation of an electron pair and the correct labeling of the Lewis acid and Lewis base? (LO 16.14) (a) (b) (c) (d)Problem 16-145
What is the pH and the principal source of H3O+ ions in 1.0 * 10-10 M HCl? (Hint: The pH of an acid solution can’t exceed 7.) What is the pH of 1.0 * 10-7 M HCl?
Problem 16.85
Calculate the pH of solutions prepared by:
(d) Mixing equal volumes of 0.20 M HCl and 0.50 M HNO3.
(Assume that volumes are additive.)
Problem 17.142
The acidity of lemon juice is derived primarily from citric acid (H3Cit), a triprotic acid. What are the concentrations of H3Cit, H2Cit-, HCit2-, and Cit3- in a sample of lemon juice that has a pH of 2.37 and a total concentration of the four citrate-containing species of 0.350 M?
Problem 17-143a
A 100.0 mL sample of a solution that is 0.100 M in HCl and 0.100 M in HCN is titrated with 0.100 M NaOH. Calculate the pH after the addition of the following volumes of NaOH:
(a) 0.0 mL
Problem 17-148e
A 40.0 mL sample of a mixture of HCl and H3PO4 was titrated with 0.100 M NaOH. The first equivalence point was reached after 88.0 mL of base, and the second equivalence point was reached after 126.4 mL of base.
(e) Sketch the pH titration curve, and label the buffer regions and equivalence points.
Problem 39c
The following pictures represent aqueous solutions of three acids HA1A = X, Y, or Z2; water molecules have been omitted for clarity.

(c) Which acid, if any, is a strong acid?
Problem 39e
The following pictures represent aqueous solutions of three acids HA1A = X, Y, or Z2; water molecules have been omitted for clarity.

(e) What is the percent dissociation in the solution of HZ?
Problem 40
Locate sulfur, selenium, chlorine, and bromine in the periodic table: (a) Which binary acid 1H2S, H2Se, HCl, or HBr2 is the strongest? Which is the weakest? Explain.Problem 41
Which of the following pictures represents a solution of a weak diprotic acid, H2A? (Water molecules have been omitted for clarity.) Which pictures represent an impossible situation? Explain.

(a) (b) (c) (d)
Problem 43a
The following pictures represent solutions of three salts NaA (A- = X-, Y-, or Z-); water molecules and Na+ ions have been omitted for clarity.
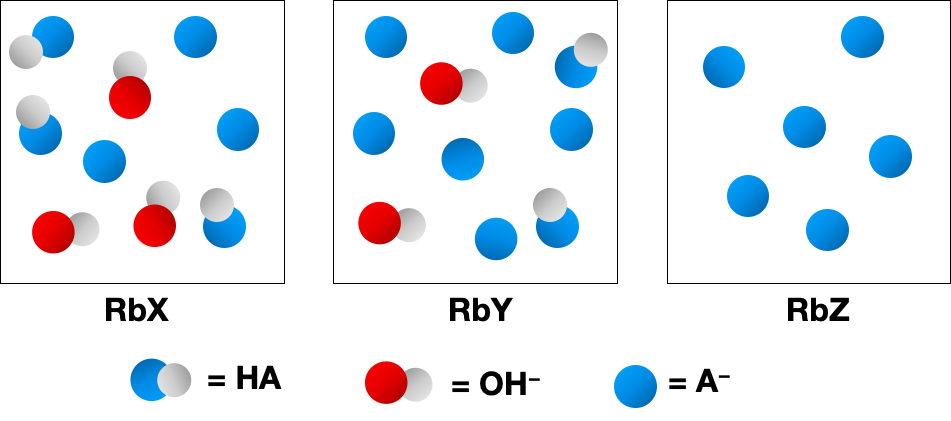
(a) Arrange the three A- anions in order of increasing base strength.
Problem 43b
The following pictures represent solutions of three salts NaA (A- = X-, Y-, or Z-); water molecules and Na+ ions have been omitted for clarity.

(b) Which A- anion has the strongest conjugate acid?
Problem 43c
The following pictures represent solutions of three salts NaA (A- = X-, Y-, or Z-); water molecules and Na+ ions have been omitted for clarity.

(c) Which A- anion has the smallest value of pKb?
Problem 44a
The following picture represents the hydrated metal cation M1H2O26 n + , where n = 1, 2, or 3.

(a) Write a balanced equation for the reaction of M1H2O26 n + with water and write the equilibrium equation for the reaction.
Problem 44b
The following picture represents the hydrated metal cation M1H2O26 n + , where n = 1, 2, or 3.
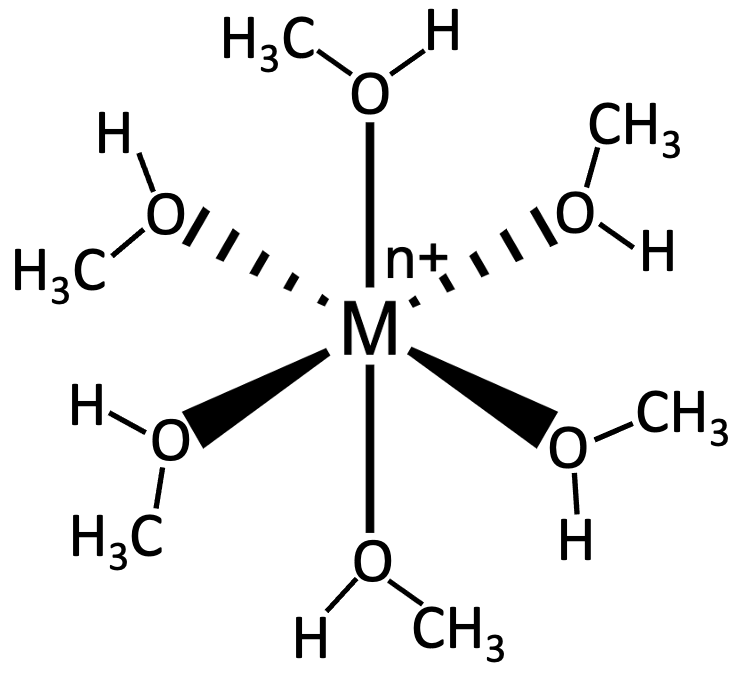
(b) Does the equilibrium constant increase, decrease, or remain the same as the value of n increases? Explain.
Problem 44c
The following picture represents the hydrated metal cation M1H2O26 n + , where n = 1, 2, or 3. (c) Which M1H2O26n + ion 1n = 1,2, or 32 is the strongest acid, and which has the strongest conjugate base?
Problem 45
Look at the electron-dot structures of the following molecules and ions: (b) Which can behave as a Lewis acid? Which can behave as a Lewis base?Problem 54
Aqueous solutions of hydrogen sulfide contain H2S, HS-, S2-, H3O+ , OH-, and H2O in varying concentrations. Which of these species can act only as an acid? Which can act only as a base? Which can act both as an acid and as a base?Problem 55
The hydronium ion H3O+ is the strongest acid that can exist in aqueous solution because stronger acids dissociate by transferring a proton to water. What is the strongest base that can exist in aqueous solution?Problem 56
Choose from the conjugate acid–base pairs HSO4- >SO42-, HF>F-, and NH4+>NH3 to complete the following equation with the pair that gives an equilibrium constant Kc 7 1. _____ + NO2 - S _____ + HNO2Problem 58
Which acid in each of the following pairs has the stronger conjugate base? See Table 16.1 to compare the relative strengths of conjugate acid-base pairs. (a) HCl or HFProblem 59
Which base in each of the following pairs has the stronger conjugate acid? See Table 16.1 to compare the relative strengths of conjugate acid-base pairs. (a) Cl- or CO32-

