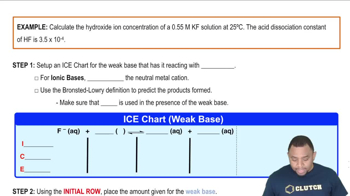
Here are the essential concepts you must grasp in order to answer the question correctly.
pH Scale
The pH scale measures the acidity or basicity of a solution, ranging from 0 to 14. A pH of 7 is neutral, below 7 indicates acidity, and above 7 indicates basicity. The pH is calculated as the negative logarithm of the hydrogen ion concentration, pH = -log[H+]. Understanding the pH scale is essential for determining the nature of the solution in the given question.
Recommended video:
Hydroxide Ion Concentration
In a basic solution, the concentration of hydroxide ions (OH-) is crucial for calculating pH. For calcium hydroxide, Ca(OH)2, it dissociates in water to produce calcium ions and hydroxide ions. The concentration of hydroxide ions can be determined from the concentration of the calcium hydroxide solution, which directly influences the pH calculation.
Recommended video:
Hydroxide Ion Concentration Example
Ion Product of Water
The ion product of water (Kw) at 25 °C is 1.0 x 10^-14, which relates the concentrations of hydrogen ions and hydroxide ions in water. This relationship is expressed as Kw = [H+][OH-]. Knowing Kw allows us to find the hydrogen ion concentration from the hydroxide ion concentration, which is necessary for calculating the pH of the solution in the question.
Recommended video:
Production of Hydrogen Example