Here are the essential concepts you must grasp in order to answer the question correctly.
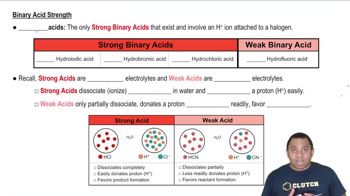
Acid Strength
Acid strength refers to the ability of an acid to donate protons (H+) in a solution. Strong acids completely dissociate in water, releasing all their protons, while weak acids only partially dissociate. The strength of an acid is often determined by its dissociation constant (Ka), with larger values indicating stronger acids.
Recommended video:
Oxidation State
The oxidation state of an element in a compound indicates the degree of oxidation (loss of electrons) of that element. In oxyacids, the oxidation state of the central atom can influence acid strength; higher oxidation states typically lead to stronger acids due to increased positive charge, which stabilizes the negative charge of the conjugate base after proton donation.
Recommended video:
Comparative Acid Strength of Oxyacids
When comparing oxyacids, the number of oxygen atoms bonded to the central atom plays a crucial role in determining acid strength. More oxygen atoms generally lead to greater acid strength because they help stabilize the negative charge on the conjugate base through resonance. Thus, among oxyacids, those with more oxygen atoms or higher oxidation states are typically stronger.
Recommended video:
Oxyacid Strength Comparison