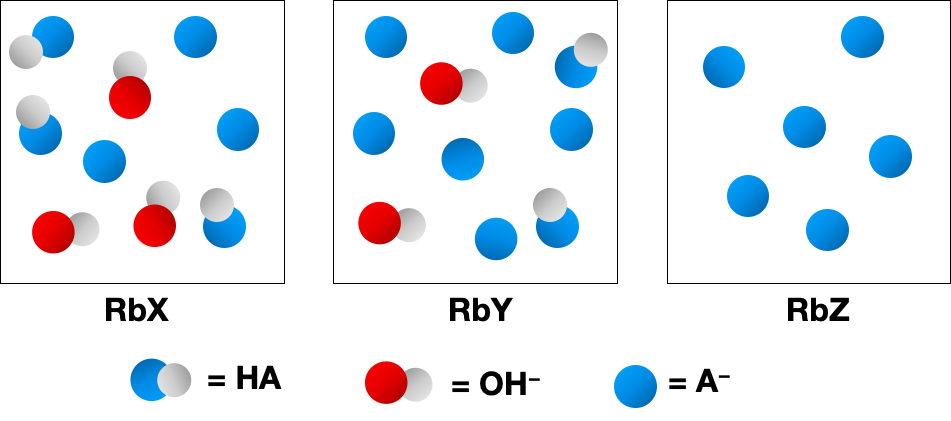
The following pictures represent aqueous solutions of three acids HA1A = X, Y, or Z2; water molecules have been omitted for clarity.
(e) What is the percent dissociation in the solution of HZ?
 Verified step by step guidance
Verified step by step guidance



The following pictures represent aqueous solutions of three acids HA1A = X, Y, or Z2; water molecules have been omitted for clarity.
(e) What is the percent dissociation in the solution of HZ?
Which of the following pictures represents a solution of a weak diprotic acid, H2A? (Water molecules have been omitted for clarity.) Which pictures represent an impossible situation? Explain.
(a) (b) (c) (d)
The following pictures represent solutions of three salts NaA (A- = X-, Y-, or Z-); water molecules and Na+ ions have been omitted for clarity.
(b) Which A- anion has the strongest conjugate acid?
The following pictures represent solutions of three salts NaA (A- = X-, Y-, or Z-); water molecules and Na+ ions have been omitted for clarity.
(c) Which A- anion has the smallest value of pKb?
The following picture represents the hydrated metal cation M1H2O26 n + , where n = 1, 2, or 3.
(a) Write a balanced equation for the reaction of M1H2O26 n + with water and write the equilibrium equation for the reaction.