20. Electrochemistry
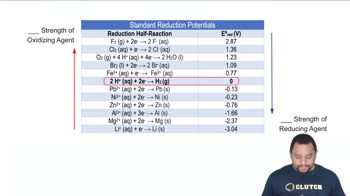
Standard Reduction Potentials
20. Electrochemistry
Standard Reduction Potentials
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
What is the oxidation number of each underlined element?
a) P4 b) BO33– c) AsO42– d) HSO4–
1412views6rank2comments - Multiple Choice
In the following reaction identify the oxidizing agent and the reducing agent:
Cr2O72- + 6 Fe2+ + 14 H+ → 2 Cr3+ + 6 Fe3+ + 7 H2O
1492views7rank5comments - Multiple ChoiceIdentify the species that is being oxidized in the following balanced reaction.
3 Pb2+ (aq) + 2 Cr (s) → 3 Pb (s) + 2 Cr3+ (aq)
670views - Multiple ChoiceWhat is the coefficient of NO when the following redox reaction is balanced in acidic solution?
NO3‾ (aq) + Cu (s) → NO (s) + Cu2+ (aq)
724views - Open Question
Wse the information in the Aleks data tab to sort the following chemical species by oxidizing power.
449views