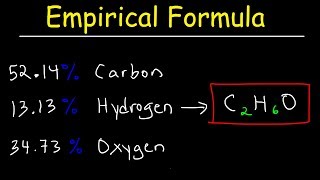
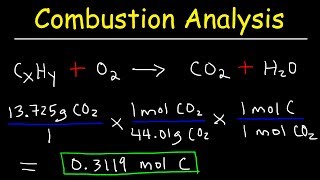
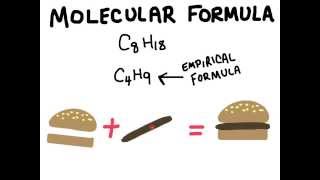
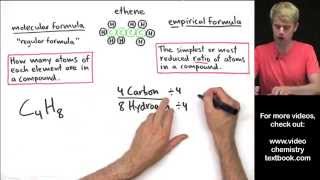
3. Chemical Reactions
Molecular Formula
3. Chemical Reactions
Molecular Formula
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
Elemental analysis of a pure compound indicated that the compound had 72.2% C, 8.50% H and the remainder as O. If 0.250 moles of the compound weighs 41.55 g, what is the molecular formula of the compound?
3054views29rank1comments - Multiple ChoiceWhich of the following is NOT a molecular compound?1351views1rank
- Multiple ChoiceWhich of the following is a molecular element?1146views
- Multiple ChoiceWhich of the following names and formulas are NOT paired correctly?789views
- Open Question
Find the molecular formula of each compound
868views - Open Question
Of the following molecular formulas for hydrocarbons which is an empirical formula
897views - Open Question
If nicotine has a molar mass of 160±5g/mol, what is its molecular formula?
799views - Open Question
A compound is 43.7% phosphorus and 56.3% oxygen. The formula mass of the compound is 284 g. calculate the empirical formula and molecular formula of the compound.
1215views