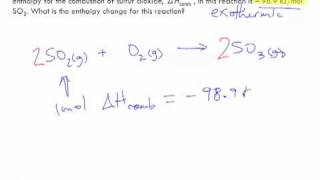8. Thermochemistry
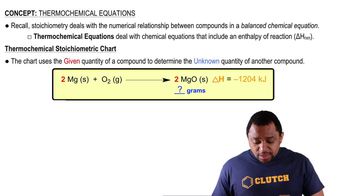
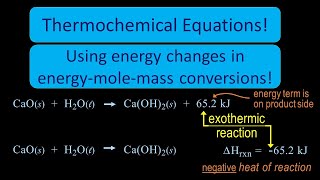
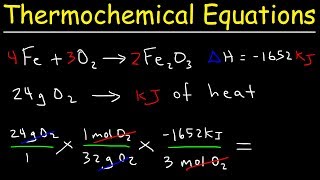
Thermochemical Equations
Learn with other creators
Practice this topic
- Multiple Choice
Nitromethane (CH3NO2), sometimes used as a fuel for drag racing, burns according to the following reaction:
4 CH3NO2 (l) + 7 O2 (g) → 4 CO2 (g) + 6 H2O (g) + 4 NO2 (g) ∆Hº = – 2441.6 kJ
How much heat is released by burning 125.0 g of nitromethane (MW:61.044 g/mol)?
4412views8rank1comments - Multiple Choice
Consider the following reaction:
2 C6H6 (l) + 15 O2 (g) → 12 CO2 (g) + 6 H2O (g) ∆Hº = – 6278 kJ
What volume of benzene (C6H6, d = 0.880 g/mL, molar mass = 78.11 g/mol) is necessary to evolve 5.19 x 109 kJ of heat?
2883views5rank - Multiple Choice
The creation of liquid methanol is accomplished by the hydrogenation of carbon monoxide:
CO (g) + 2 H2 (g) → CH3OH (l) ∆Hº = – 128.1 kJ
How much heat (in kJ) is released when 125.0 g CO reacts with 2.32 x 102 g H2?
4878views10rank1comments - Multiple ChoiceConsider two identical iron nails: One nail is heated to 95℃, the other is cooled to 15℃. The two nails are placed in a coffee cup calorimeter and the system is allowed to come to thermal equilibrium. What is the final temperature of the two nails?775views
- Open Question
Which answer best describes the transfer of heat that occurs when 1.50 mol of H2 reacts?
833views