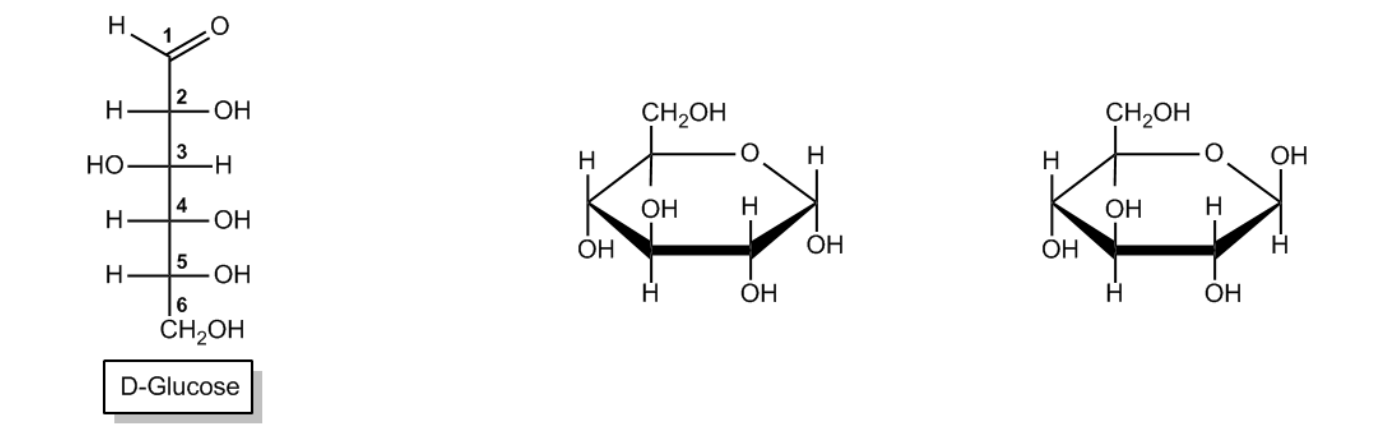
Now we're going to talk about another type of representation that's very important for sugar chemistry, and that's the Haworth projection. So guys, Haworth projections are simplified drawings of sugars in a cyclic form, imagining that your ring is planar. Now we know that it's not planar. We know that it's in a puckered chair conformation according to the rules of cyclohexane that we learned in Orgo 1. But we use these simplified structures a lot because they're just easier to draw. Okay?
Now, what do you need to know about converting a monosaccharide chain into a Haworth? Guys, it's exactly like a cyclohexane, just easier. The anomeric carbon, note, is always at the far right in a Haworth projection, which is exactly the same as cyclohexane. Your anomeric position, your one position should always be there. So that means this carbon is the same as that carbon there. And in the same way, you're going to number going clockwise. So it's going to be 2, 3, 4, 5, and remember this is actually 6. Okay? So let's say this is true for both pyranose and furanose rings. So in this case, these are pyranose rings, but if there were furanose rings, your anomeric carbon should still go to the right.
Now, this practice problem is pretty straightforward. It says properly number the following Haworth projections, identify each anomer, and predict the anomeric equilibrium. Well, guys, I already started on this problem before we even began because I started showing you guys how your right carbon should be number 1, right? It should be your anomeric. So that means that this is your 1 and that you should just go clockwise like you would with this regular cyclohexane, meaning that this is also 1, 2, 3, 4, 5, 6. Okay?
Now, can you guys explain to me why carbon 6 is going up? Because once again, we have a D-glucose, which is like a D-stereo isomer, and D always goes up with the stereo descriptor always goes up, okay? Now, how can we identify each anomer? So notice that this O is facedown and this O is faced up. So how do we figure out which one's alpha and which one's beta? Well, notice that for both of these, the stereo descriptor is faced up meaning that this is a trans and this is a cis. So guys, the same rules are going to apply. This is the alpha anomer. And this is the beta anomer. Okay?
Now guys, I told you you're not going to be responsible to know when alpha is more prevalent over beta, at least not now. That you need more knowledge for that. But I did already show you guys what the anomeric equilibrium was specifically for D-glucose, and remember which one was favored. Remember it was the beta, right? It was actually the beta. So I'm going to go ahead and draw that the anomeric equilibrium is more towards the beta and less towards the alpha simply because I already showed you the same exact molecule. Okay? Just because it is this way for mannose it might be different. But for glucose, we know that one specifically, we know that it's going to go to the right. Okay? Awesome guys, that wasn't so bad right? Now you have another even easier way to represent monosaccharides. Let's move on to a practice problem.