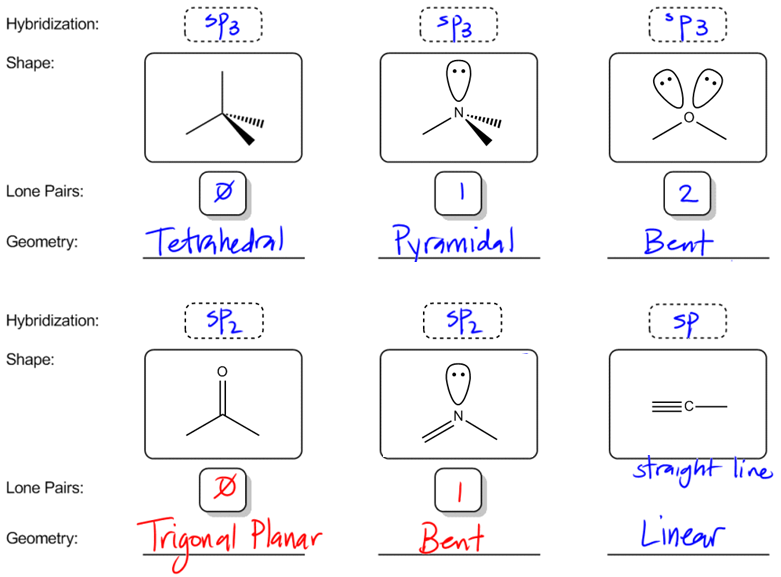
So now let's bring this all together talking about molecular geometry and VSEPR theory. So, VSEPR theory is something that you should have learned in gen chem, and what I just want to draw a distinction. When I talk about hybridization, I'm always talking about the orbitals that are hybridizing, that are blending. So sp_3, sp, whatever. When I talk about geometry, what I'm going to be talking about is the actual shape of the molecule. What does it look like if I were to put it under a microscope? What kind of shape would it have? Alright. So let's go ahead and start off with these first three molecules. I'll just make them 1, 2, 3. And what I want you guys to do is use the hybridization summary chart and use the rules that I taught you of bond sites to figure out what kind of hybridization these 3 will have. So go ahead and pause the video. Try to figure out the hybridization for all 3.
Alright. So if you were using the rules correctly, what you would have noticed is that these are all sp3 hybridized. Even though they look very different, they're all sp3 hybridized. And the reason why is because they all have 4 bond sites. Some of them have 4 atoms. Some of them have atoms and lone pairs, but that's still 4 bond sites total. Alright. So now what I want to do is enter in the amount of lone pairs that each one has. So as you can see, this first one doesn't have any lone pairs. So I'm just going to put 0. The second one has one lone pair and the third one has 2 lone pairs. Oh my gosh, 2. Alright. So I have 1, 2 and you can see that all these are sp3 hybridized, but they all look different. And that's where the geometry comes in. Even though they're all sp3 hybridized, we want words that are going to distinguish something that looks like 1. 1 is very different looking from 3. We want words that are going to describe those shapes. So the name that I'm going to use for 0 lone pairs, does anyone know? I think I heard you say it. Tetrahedral. You've heard of this forever. Well, at least since gen chem. The tetrahedral is the name given to the shape of 4 bond sites with 0 lone pairs. So every bond site is an atom or is a bond. Now if I have one lone pair, what I'm going to envision is that think about for all these names, think about that I can't see the lone pairs. Remember that electrons are tiny. So imagine that I'm looking with a microscope and I can't see the lone pair at all. What is this going to look like? Well, if you think about it, it kind of looks like a pyramid. Right? Think about it. This is like the top of the pyramid. This is like Giza or whatever. So the name of this the full name is trigonal pyramidal or what sometimes is just called pyramidal. Trigonal pyramidal. but most of the time it's just called pyramidal. Alright guys, so that's just the name of the shape. And then finally, what if we have 2 lone pairs? That means there's even less that I can see. So when I'm just looking at this, all I see is something get...
...Awesome. So now what I want to do is move on to other types of hybridization. So what if I have this atom right here and this atom right here? I want you guys to also pause the video and figure out what kind of hybridizations these 2 would have. So let's call these 4, 5. Figure out what their hybridizations would be. Alright. So both of these, because they have 3 bond sites each, are going to be sp2. Let me point out how it's 3. For the double bond O, I have basically 1 atom here, 1 atom here, 1 atom here. That's 3. For the n, I have 1 atom here, 1 atom here, and a lone pair there, which is also 3. Get it? So they're both sp2. But then they also look different. They don't look the same as each other. Notice that one of these has 3 things, 3 atoms coming off of it. The other one only has 2. So if I were to talk about lone pairs, the lone pairs would be 0 here and 1 here. It turns out that these oftentimes will get different names as well. So the name of this first one, just so you guys know, if you have an sp2 with 0 is trigonal, by the way, this is the same way that you spell trigonal for trigonal pyramidal. In case you wanted to spell out trigonal pyramidal, it would be this word, trigonal planar. Trigonal planar is the name of it. The reason why is because when you have an sp2 hybridized atom, all of the groups are going to be on the same plane. So in fact, if you thought about it, the way that I like to think of sp2 is kind of like a sand dollar, where imagine that this is like you're walking on the beach and you see this sand dollar and you pick it up. That's basically the way trigonal planar looks where you have those three lines, a line here, a line here, a line here, and they're all on the same plane. It's just a very flat object. It has two sides. So that's trigonal planar. Does that make sense so far? Cool. Now what about if it has one lone pair? Now, this actually is a little bit controversial, and I've been looking for a universal answer to this question, but really it turns out that it just varies by professor. So I'm just going to tell you this. Some professors are going to call this bent because really, if you take out that lone pair, it looks bent. Does that make sense so far? So some professors are going to say that that's bent, but then other professors are actually just going to say both of these are trigonal planar. I'm going to put here... Alright. They're just going to say, Hey, both of them are trigonal planar. Don't really worry about the lone pairs. Alright. So I'm just going to teach it to you both ways and just tell you guys to be aware, to be mindful when your professor says that. Maybe you even want to ask them. Would you consider this molecule trigonal planar or would you consider it bent? You can also see the answers to practice problems, however they answer them. That will tell you. So I just wanted to let you know those are the 2 different ways of looking at it. Technically, trigonal planar is always correct. If you say that that's trigonal planar, that should be correct, but bent is just a little bit more specific and some professors want you to be that specific. Cool? So then finally, we have this last one, which is really easy. A carbon I'm not even going to make you guys pause it. A carbon with 2 groups that should be what? That should be sp. In this situation, I don't worry about lone pairs or not lone pairs. It's always just going to get the same name and that is linear. This is why it's very important whenever you're drawing triple bonds, it's very, very important that you always draw triple bonds in a straight line. In fact, if you draw your triple bond bent like with a zigzag, you will get points off taken off your test. Okay? And the reason is because it makes it look like you don't know what you're doing. It makes it look like you have no clue that that's supposed to be a linear hybridization or a linear shape. So many times when you see a triple bond written, you're going to see it like this. 1, 2 and then like a triple bond like that. And that means that's right over my head. But that means that you're indicating that hey, this has a bond angle of 180 degrees and it's linear. Cool?