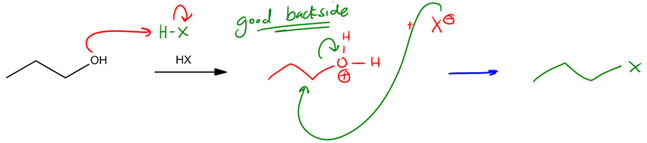
As you guys might have figured out by now, alcohols are a pretty important functional group for organic chemistry, but there's one major limitation of alcohols and that's that they make terrible leaving groups. Okay? Remember that the definition of a good leaving group is something that once it leaves, it's stable. Well, alcohol after it leaves, it becomes OH-. OH- is the same as a hydroxide base, which is a very unstable molecule. It's a very strong base. So that means that whenever we have an alcohol, we're a little bit stuck. We don't know exactly what to do with it because a lot of reactions in organic chemistry require leaving groups and alcohol isn't a good option. But wait, there is a solution. It turns out that a major topic that we're going to discuss in this section is how to turn alcohol into a good leaving group.
It turns out that there are 2 major options like a fork in the road. We can take 2 major pathways, and they're both going to lead to awesome outcomes. They're both going to lead to alcohol being a much better leaving group. Let's go ahead and talk about the first one. The first option that we have is to convert alcohol simply into an alkyl halide. Now remember that alkyl halides have the molecular formula RX, and the reason that they're such good leaving groups is because X-, once it takes off, is very stable. X could stand for iodine or bromine or chlorine. These are very electronegative atoms that don't mind having a negative charge. So alkyl halides are an awesome option. But another option that we also have that we'll discuss in a little bit is sulfonate esters. Now you might have learned already that sulfonate esters make great leaving groups. I actually have talked about that before, but now what we're going to learn is how to actually turn an alcohol into a sulfonate ester so that it can become a great leaving group. Okay?