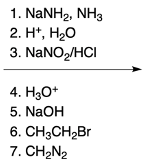
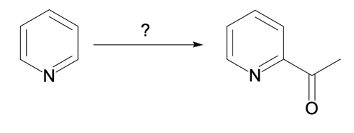
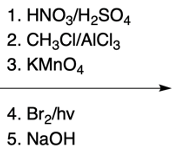
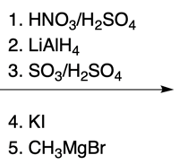
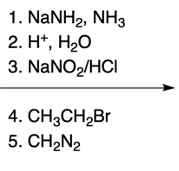
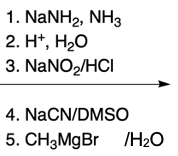
Hey, everyone. So in this video, we're going to talk about SNAr reactions of our pyridine. Now when we say SNAr, that stands for nucleophilic aromatic substitution. Here, we're going to say that the electron deficiency of pyridine makes it more susceptible to nucleophilic aromatic substitution than benzene. And we're going to say two major NAS or nucleophilic aromatic substitution reactions of pyridine involve substitution on the ortho position.
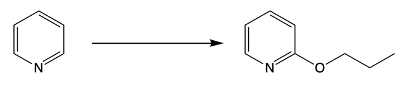
Here we have reaction 1, which is called the Chichibabin reaction. Now this is typical of our SNAr reaction of NH2 onto the pyridine ring. And then we have an organometallic reaction, where this is an SNAr of an alkyl anion onto the pyridine ring. Right now, let's look at the first reaction, our Chichibabin reaction. We're going to say that this is a method to create 2-aminopyridine by reacting pyridine with sodium amide.
Remember, sodium amide represents a strong base. So here we have our pyridine, and we're looking at this hydrogen here. Through the use of sodium amide over our ammonia solvent, followed by H+ and water, which we could think of as acidic hydrolysis, we would have our hydrogen being replaced with an NH2. This would make our 2-aminopyridine product, and then we'd have our hydrogens.
So this blue hydrogen here with another hydrogen to give us an H2. This H2 would just be a byproduct. What's more important is that we made a 2-aminopyridine through the Chichibabin reaction. Now recall when we talk about nucleophilic aromatic substitution, it occurs by an addition-elimination mechanism. So when we look at the mechanism for this reaction, we're going to remember some of the NAS reactions we've seen before in the past.
Alright. So it's going to be stuff that's not exactly new, but we're just applying it now to make a 2-aminopyridine product.