Hey guys. In this video, we're going to discuss common fragmentation patterns found within mass spectrometry. So basically, we're going to discuss the step of ionization and everything that follows after that. Before we can truly understand why some fragments are going to be more favored than others, we need to grasp this concept called ionization potential. Ionization potential tells us how likely an electron is to be knocked off by an electron beam. It turns out guys that not all electrons are made equal. Some of them are going to be held very tightly by molecules, so they are more difficult to knock off. And some of them are going to be held very loosely, so that means they are the first ones to go when they hit this electron beam. These patterns, these ionization potentials can help us to predict what is going to be the major cation that is formed or what is going to be the major radical cation that is formed after ionization has taken place.
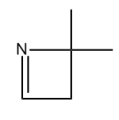
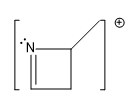
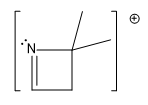
Guys, here I've just made a very simple trend that I'd love for you guys to memorize. And all it is this, that basically one of the easiest ones to knock off is the lone pair of a nitrogen. And guys, this is for the same reason that we've kind of always thought of the nitrogen as having a very reactive lone pair. It's very loosely held. It's very easy to ionize that lone pair. So if you have a nitrogen with a lone pair on your sample, that lone pair is the most susceptible.
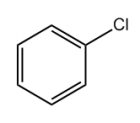
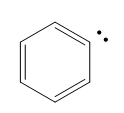
Now in terms of this kind of spectrum, we're just going to kind of go down one by one the different types of compounds that get a little bit harder to ionize with every step. The next one would actually be aromatics. Aromatics, these are the general category of benzene and benzene-like molecules. And it turns out that any of the single bonds directly attached to an aromatic are actually relatively easy to ionize. Aromatics tend to give a radical cation that doesn't involve breaking the ring because that ring is very stable. So we want to keep that ring intact. We end up just breaking off one of the ends, one of the single bonds attached to it that is coming off of it. In terms of ionization, we wouldn't ionize the actual bonds of the ring. We would ionize maybe one of the hydrogens or something that is attached to the benzene ring.
For similar reasons, double bonds come next. This is what we call vinyl. A vinyl position means directly attached to a double bond. It's a little bit harder than benzene, but it's still not that bad. We would prefer to knock off a vinyl position, something that is on a double bond rather than something that is not on a double bond. Then we get lone pairs attached to oxygen for similar reasons as nitrogen, just less reactive, less susceptible. So this one would have a higher ionization potential.
And finally, the hardest one, guys, the ion of last resort would simply be a single bond that is attached to other single bonds. In this case, notice that I'm not attached to a double bond. I'm not attached to a ring. I mean, I'm not attached to a benzene. I'm just attached to something that is an alkane. Now you might be wondering Johnny, does it matter that it's a ring? No. I'm just using rings here to keep everything consistent because what I'm trying to show you is that it's not the ring that matters, it's really the stability of that ring, whether it's a benzene, whether it's a double bond or whether it's just an alkane. An alkane, similar to the methane that we used in our intro video, would be one of the more difficult ones to ionize because there's really nothing helping those radicals to get loose. That's just going to take brute force to remove one of those electrons and make the radical cation. There's no extra stabilizing factors for an alkane.
All right. Awesome guys. So that's ionization potentials. Now let's move on to simple fragmentation mechanisms.