Here are the essential concepts you must grasp in order to answer the question correctly.
Alcohols and Their Functional Groups
Alcohols are organic compounds characterized by the presence of one or more hydroxyl (-OH) functional groups. This functional group is responsible for the unique properties of alcohols, including their ability to engage in hydrogen bonding, which affects their boiling points and solubility. Understanding the structure of alcohols is crucial for predicting their reactivity in chemical reactions.
Recommended video:
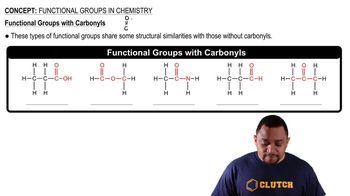
Carbonyl Functional Groups
Substitution Reactions
Substitution reactions involve the replacement of one atom or group in a molecule with another atom or group. In the context of alcohols reacting with sodium, the hydroxyl group (-OH) is replaced by a sodium ion (Na+), resulting in the formation of an alkoxide ion. This type of reaction is fundamental in organic chemistry, as it helps in the synthesis of various compounds.
Recommended video:
Alcohol Reactions: Substitution Reactions
Reactivity of Sodium with Alcohols
Sodium reacts vigorously with alcohols, leading to the formation of alkoxides and the release of hydrogen gas. This reaction is an example of a metal reacting with an alcohol, where sodium donates an electron to the hydroxyl group, facilitating the substitution. Understanding this reactivity is essential for predicting the products of alcohol reactions and their applications in organic synthesis.
Recommended video:
Rules for Naming Alcohols

 Verified step by step guidance
Verified step by step guidance