Here are the essential concepts you must grasp in order to answer the question correctly.
Alcohol Functional Group
Alcohols are organic compounds characterized by the presence of one or more hydroxyl (-OH) groups attached to a carbon atom. This functional group is responsible for the unique chemical properties of alcohols, including their ability to participate in various reactions such as oxidation, dehydration, and substitution.
Recommended video:
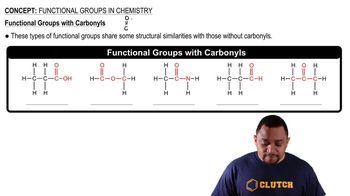
Carbonyl Functional Groups
Types of Reactions Involving Alcohols
Alcohols can undergo several types of chemical reactions, including oxidation (where they are converted to aldehydes, ketones, or carboxylic acids), dehydration (leading to the formation of alkenes), and substitution reactions (where the -OH group is replaced by another functional group). Understanding these reactions is crucial for predicting the products formed.
Recommended video:
Alcohol Reactions: Dehydration Reactions
Reaction Mechanisms
The mechanisms of alcohol reactions involve specific steps that describe how reactants are transformed into products. For example, in oxidation, the mechanism may involve the formation of a carbocation intermediate, while dehydration typically involves the removal of water. Familiarity with these mechanisms helps in understanding the conditions required for each reaction and the expected products.
Recommended video:
Reaction Mechanism Overview