Here are the essential concepts you must grasp in order to answer the question correctly.
Alcohol Functional Group
Alcohols are organic compounds characterized by the presence of one or more hydroxyl (-OH) groups attached to a carbon atom. This functional group is responsible for the unique chemical properties of alcohols, including their ability to participate in various reactions such as oxidation, dehydration, and substitution.
Recommended video:
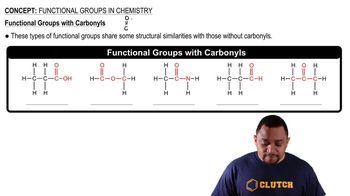
Carbonyl Functional Groups
Types of Reactions Involving Alcohols
Alcohols can undergo several types of chemical reactions, including oxidation, where they are converted to aldehydes or ketones, and dehydration, which leads to the formation of alkenes. Additionally, alcohols can react with acids to form esters through esterification, showcasing their versatility in organic chemistry.
Recommended video:
Alcohol Reactions: Dehydration Reactions
Reaction Products
The products of alcohol reactions depend on the type of reaction and the structure of the alcohol. For example, primary alcohols oxidize to aldehydes, while secondary alcohols oxidize to ketones. Understanding the specific reaction conditions and the nature of the alcohol is crucial for predicting the correct products formed in each case.
Recommended video:
Production of Hydrogen Example