Here are the essential concepts you must grasp in order to answer the question correctly.
Aldehydes
Aldehydes are organic compounds characterized by the presence of a carbonyl group (C=O) at the end of a carbon chain. The general formula for aldehydes is RCHO, where R represents a hydrocarbon group. They are named by replacing the '-e' ending of the corresponding alkane with '-al'. For example, the simplest aldehyde is formaldehyde (methanal), derived from methane.
Recommended video:
Rules for Naming Aldehydes
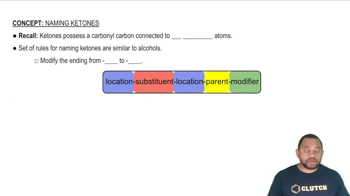
Ketones
Ketones are organic compounds that contain a carbonyl group (C=O) located within a carbon chain, specifically between two carbon atoms. The general formula for ketones is RC(=O)R', where R and R' are hydrocarbon groups. Ketones are named by replacing the '-e' ending of the corresponding alkane with '-one'. For instance, propanone (acetone) is the simplest ketone derived from propane.
Recommended video:
Nomenclature of Organic Compounds
The nomenclature of organic compounds follows specific rules set by the International Union of Pure and Applied Chemistry (IUPAC). This system involves identifying the longest carbon chain, determining functional groups, and applying appropriate suffixes and prefixes. For aldehydes and ketones, the position of the carbonyl group is crucial, and numbering the carbon chain helps in naming the compound accurately.
Recommended video:
Introduction to Organic Chemistry