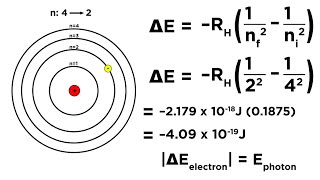
9. Quantum Mechanics
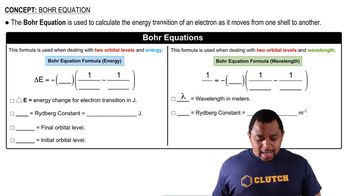
Bohr Equation
Learn with other creators
Practice this topic
- Multiple Choice
What is the wavelength of a photon (in nm) absorbed during a transition from the n = 2 to n = 5 state in the hydrogen atom?
2541views7rank2comments - Multiple Choice
Determine the end (final) value of n in a hydrogen atom transition, if the electron starts in n = 5 and the atom releases a photon of light with an energy of 4.5738 × 10-19 J.
3019views1rank1comments - Multiple Choice
An electron releases energy as it moves from the 6th shell to the 3rd shell. If it releases 4.25 x 109 kJ of energy at a wavelength of 915.7 nm, how many photons were released in the process?
1849views2rank4comments - Open Question
Calculate the frequency of the light emitted when an electron in a hydrogen atom makes each of the following transitions.
642views - Open Question
Which wavelength in the hydrogen emission spectrum corresponds to the transition from n=5 to n=2?
854views - Open Question
If our eyes could see a slightly wider region of the electromagnetic spectrum, we would see a fifth line in the Balmer series emission spectrum. Calculate the wavelength λ associated with the fifth line.
804views - Open Question
Calculate the energy of a photon emitted when an electron in a hydrogen atom undergoes a transition from 𝑛=6 to 𝑛=1.
907views