7. Gases
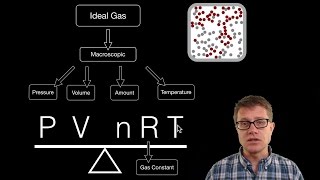
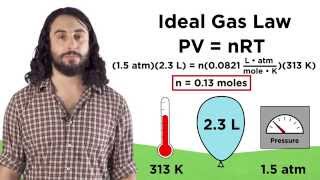
The Ideal Gas Law
7. Gases
The Ideal Gas Law
Additional 5 creators.
Learn with other creators
Showing 8 of 8 videos
Practice this topic
- Multiple Choice
How many grams of carbon dioxide, CO2, are present in a 0.150 L flask recorded at 525 mmHg and 32 ºC?
2652views22rank3comments - Multiple Choice
How many liters of HNO3 gas, measured at 28.0 ºC and 780 torr, are required to prepare 2.30 L of 4.15 M solution of nitric acid?
2202views16rank2comments - Multiple Choice
When 0.670 g argon is added to a 500 cm3 container with a sample of oxygen gas, the total pressure of the gases is found to be 1.52 atm at a temperature of 340 K. What is the mass of the oxygen gas in the bulb?
2629views14rank1comments - Multiple ChoiceA container with a movable piston contains 2.0 moles of argon gas. When the volume of the container is 1.5 L, the pressure is 2.4 atm. Calculate the pressure of the container when the piston is raised to give a volume of 2.5 L. Assume the temperature remains constant.965views
- Open Question
What is the temperature of 0.750 mol of a gas stored in a 6,850 mL cylinder at 2.21 atm?
628views - Open Question
What is the temperature of 0.80 mol of a gas stored in a 275 mL cylinder at 175 kPa?
763views - Open Question
What pressure will 14.0 g of CO exert in a 3.5 L container at 75°C?
928views - Open QuestionWould the volume be different if the gas was argon (under the same conditions)?810views