6. Chemical Quantities & Aqueous Reactions
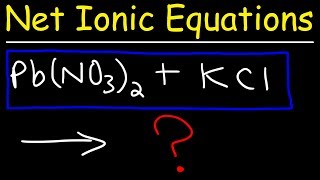
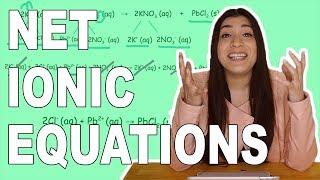
Complete Ionic Equations
Learn with other creators
Practice this topic
- Multiple Choice
Provide the net ionic equation that occurs when the following aqueous compounds are mixed together:
Copper (II) Bromide and Lithium Hydroxide
801views6rank4comments - Multiple Choice
Which of the following reagents could be used to separate the two anions from a solution containing magnesium nitrate and cesium hydroxide?
735views5rank - Multiple Choice
Which of the following reagents could be used to separate the two cations from a solution containing Lead (IV) acetate and cesium permanganate?
762views2rank - Multiple ChoiceAn unknown sample is analyzed using the general qualitative analysis scheme shown in Figure 18.16. The sequence of reagents shown in the figure are used and a precipitate in only produced when (NH4)2HPO4 in NH3 is added. Which of the following describes a set of ions that might be present in the solution?365views
- Open Question
What is the net ionic equation of the reaction of FeCl2 with NaOH?
287views - Open Question
Give the complete ionic equation for the reaction (if any) that occurs when aqueous solutions of lithium sulfide and copper (II) nitrate are mixed.
293views2rank - Open Question
Write the balanced net ionic equation for the reaction that occurs in the following case: Ba(NO3)2(aq) + K2SO4(aq)→
368views - Open Question
Enter the net ionic equation representing aqueous acetic acid neutralized by aqueous barium hydroxide.
291views