6. Chemical Quantities & Aqueous Reactions
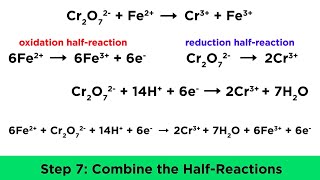
Balancing Redox Reactions: Basic Solutions
6. Chemical Quantities & Aqueous Reactions
Balancing Redox Reactions: Basic Solutions
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
Balance the following redox reaction in a basic solution
H2O2 (aq) + ClO2 (aq) → ClO2- (aq) + O2 (g)
858views4rank1comments - Multiple Choice
Balance the following redox reaction in a basic solution.
ClO2− (aq) → Cl- (aq) + ClO4− (aq)
1746views9rank1comments - Open Question
Balance the following redox reaction if it occurs in basic solution. What are the coefficients in front of Br2 and OH- in the balanced reaction?
Br2(l) → BrO3-(aq) + Br(aq)
240views - Multiple ChoiceWhich of the following is the correctly balanced redox reaction for H2O2(aq) + ClO2(aq) → ClO₂⁻(aq) + O₂(g) in a basic solution?
- Multiple ChoiceWhich of the following is the correctly balanced redox reaction for MnO4⁻(aq) + Br⁻(aq) → MnO2(s) + BrO3⁻(aq) in a basic solution?