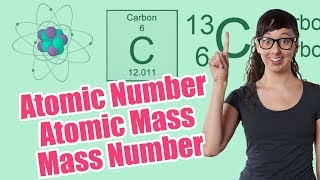2. Atoms & Elements
Atomic Mass
Learn with other creators
Practice this topic
- Multiple Choice
Which of the following choices has the greatest atomic mass?
3245views18rank1comments - Multiple Choice
Neon possesses three naturally occurring isotopes. 20Ne has a percent abundance of 90.48% and an isotopic mass of 19.99244 amu, 21Ne has a percent abundance of 0.27% and an isotopic mass of 20.99384 amu, and 22Ne has a percent abundance of 9.25%. What is the isotopic mass of the 22Ne isotope?
1369views - Multiple Choice
Three isotopic forms of potassium exist: 39K, 40K and 41K. Potassium has an atomic mass of 39.0983 amu. Potassium-40 has an isotopic mass of 39.9640 amu and natural abundance of 0.0117%. Potassium-41 has an isotopic mass of 40.9618 amu and natural abundance of 6.7302%. What is the isotopic mass of Potassium-39?
2358views9rank1comments - Multiple Choice
Neon possesses three naturally occurring isotopes. 20Ne has a percent abundance of 90.48% and an isotopic mass of 19.99244 amu, 21Ne has a percent abundance of 0.27% and an isotopic mass of 20.99384 amu, and 22Ne has a percent abundance of 9.25%. What is the isotopic mass of the 22Ne isotope?
1199views11rank - Open Question
Boron has two isotopes, boron-10 and boron-11, whose percentage abundances are 19.8% and 80.2% respectively. the atomic masses of boron-10 and boron-11 are 10.0129 amu and 11.0093 amu respectively. write the symbols for the two isotopes of boron and determine the relative atomic mass.
1734views - Open Question
Boron obtained from borax deposits in death valley consists of two isotopes. They are boron-10 and boron-11 with atomic masses of 10.013 amu and 11.009 amu, respectively. The atomic mass of boron is 10.81 amu (see periodic table). Which isotope of boron is more abundant, boron-10 or boron-11?
937views - Open Question
Lithium has an elemental atomic mass of 6.941 u and has two naturally occurring isotopes, 6Li and 7Li. Their masses are 6.0151 u and 7.0160 u respectively. What are the natural abundances (to 2 decimal places in percentage) of the isotopes of lithium?
1793views - Open Question
Calculate the atomic mass of element ""X"", if it has 2 naturally occurring isotopes with the following masses and natural abundances:
X-45 44.8776 amu 32.88%
X-47 46.9443 amu 67.12%.
1278views