15. Chemical Kinetics
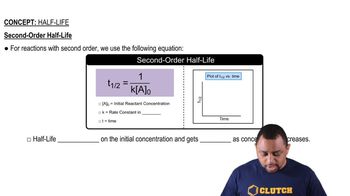
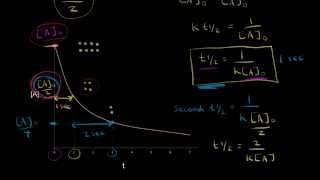
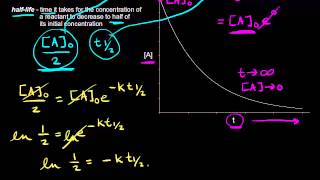
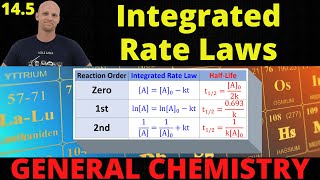
Half-Life
Learn with other creators
Practice this topic
- Multiple Choice
Decomposition of a certain substance Y at 45°C was found to be zero order. What is the half-life of substance Y if it took 15.5 minutes to decompose 67% of this substance? [Y]0 = 0.25 M.
1125views2rank5comments - Multiple Choice
Radioactive plutonium-239 (t1/2 = 2.41 × 105 yr) is used in nuclear reactors and atomic bombs. If there are 5.70 × 102 g of plutonium isotope in a small atomic bomb, how long will it take for the substance to decay to 3.00 × 102 g?
1932views4rank1comments - Multiple Choice
Use the data below to determine the half-life of decomposition of NOCl reaction which follows 2nd order kinetics.
1183views4rank4comments - Multiple ChoiceThe conversion of methyl isonitrile into acetonitrile follows first-order kinetics with a half-life of 110.2 seconds. What mass of a 1.00-g sample of methyl isonitrile remains after 10.0 minutes?
CH3 – N≡C → CH3 – C≡N906views - Open Question
How long will it take for the concentration of SO2Cl2 to decrease to 25% of its initial concentration?"
823views - Open Question
What is the rate constant of a first-order reaction when 20.0% of a reactant remains after 30.0 s?
645views - Open Question
What is the half-life for a particular reaction if the rate law is rate = (1301 min-1)[A]?
654views