15. Chemical Kinetics
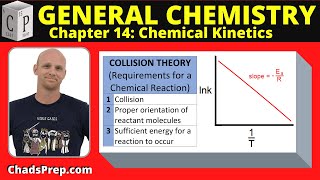
Collision Theory
15. Chemical Kinetics
Collision Theory
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Open Question
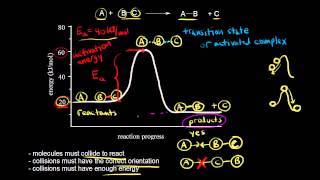
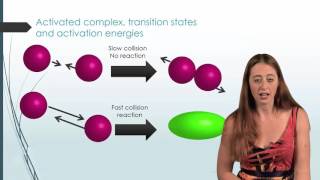
What must happen before a chemical reaction can begin? The activation energy must be exceeded. The activation energy must be reached. The concentrations of products and reactants must be equal. The concentration of reactant molecules must be reduced.
535views - Open Question
What factors determine whether a collision between two reactant molecules will result in a reaction?
518views - Open Question
What must happen before a chemical reaction can begin?
550views - Open Question
What must happen before a chemical reaction can begin? The activation energy must be exceeded. The activation energy must be reached. The concentrations of products and reactants must be equal. The concentration of reactant molecules must be reduced.
495views - Multiple ChoiceWhich statement correctly applies to the collision theory of chemical reactions?34views
- Multiple ChoiceWhich statement best describes collision theory in chemistry?43views
- Multiple ChoiceWhich statement correctly expresses collision theory?40views
- Multiple ChoiceAccording to the collision theory, which of the following is necessary for a chemical reaction to occur between two molecules?44views