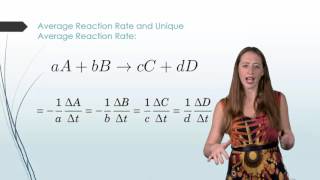15. Chemical Kinetics
Average Rate of Reaction
15. Chemical Kinetics
Average Rate of Reaction
Additional 2 creators.
Learn with other creators
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
Consider the following reaction:2 H2O2 (aq) → 2 H2O (l) + O2 (g)
Calculate the rate of the reaction between 25 sec and 65 sec.
2416views34rank4comments - Multiple ChoiceThe rate of consumption of C3H6 in the following reaction is 0.45 M/s. Calculate the rate of production of H2O.
2 C3H6 + 2 NH3 + 3 O2 → 2 CH2CHCN + 6 H2O635views - Open Question
Which species has the greatest rate of appearance in the reaction below? 2 H2S + O2 → 2 S + 2 H2O
568views - Open Question
Which species has the greatest rate of disappearance in the reaction below? CH4 + 2 O2 → CO2 + 2 H2O
640views - Open Question
Which species has the greatest rate of disappearance in the reaction below? CH4 + 2 O2 → CO2 + 2 H2O
488views - Multiple ChoiceConsider the following reaction:2N2O(g) → 2N2(g) + O2(g)In the first 11.0 s of the reaction, 0.015 mol of O2 is produced in a reaction vessel with a volume of 0.500 L. What is the average rate of the reaction during this time interval?280views
- Multiple ChoiceIn a chemical reaction where A is being converted to B, if the concentration of [A] at t₀ = 0.325 M and 35 seconds later [A] = 0.145 M, what is the average rate of reaction over the 35-second time interval?353views