14. Solutions
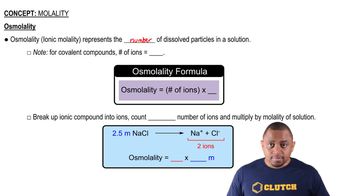
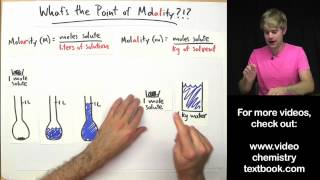
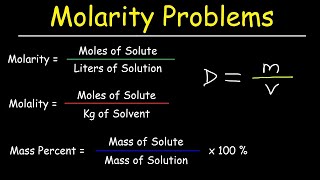
Molality
Learn with other creators
Practice this topic
- Multiple Choice
A solution is prepared by dissolving 43.0 g potassium chlorate, KClO3, in enough water to make 100.0 mL of solution. If the density of the solution is 1.760 g/mL, what is the molality of KClO3 in the solution?
2109views14rank - Multiple Choice
The density of a 15.7 M methanol (CH3OH) solution is 0.858 g/mL. If H2O is the solvent, what is the molality of the solution?
2553views13rank4comments - Multiple Choice
What is the ionic molality of sodium ions in a solution of 25.7 g NaNO3 dissolved in enough water to make a 150.0 mL of solution? Density of the solution is 1.02 g/mL.
1612views12rank4comments - Multiple ChoiceWhat is the molarity of a solution prepared by dissolving 5.00 g of NaCl in 500.0 mL of water?1070views
- Open Question
What mass (in g) of nh3 must be dissolved in 475 g of methanol to make a 0.250 m solution?
1173views - Open Question
What is the molar concentration of Na+ ions in 0.0250 m solutions of the following sodium salts in water?
839views - Open Question
Commercial grade HCl solutions are typically 39.0% (by mass) HCl in water. Determine the molality of the HCl, if the solution has a density of 1.20 g/mL.
680views - Open Question
If we dissolve 36 grams of salt in 148 grams of water, what is the mass of the resulting solution?
777views