14. Solutions
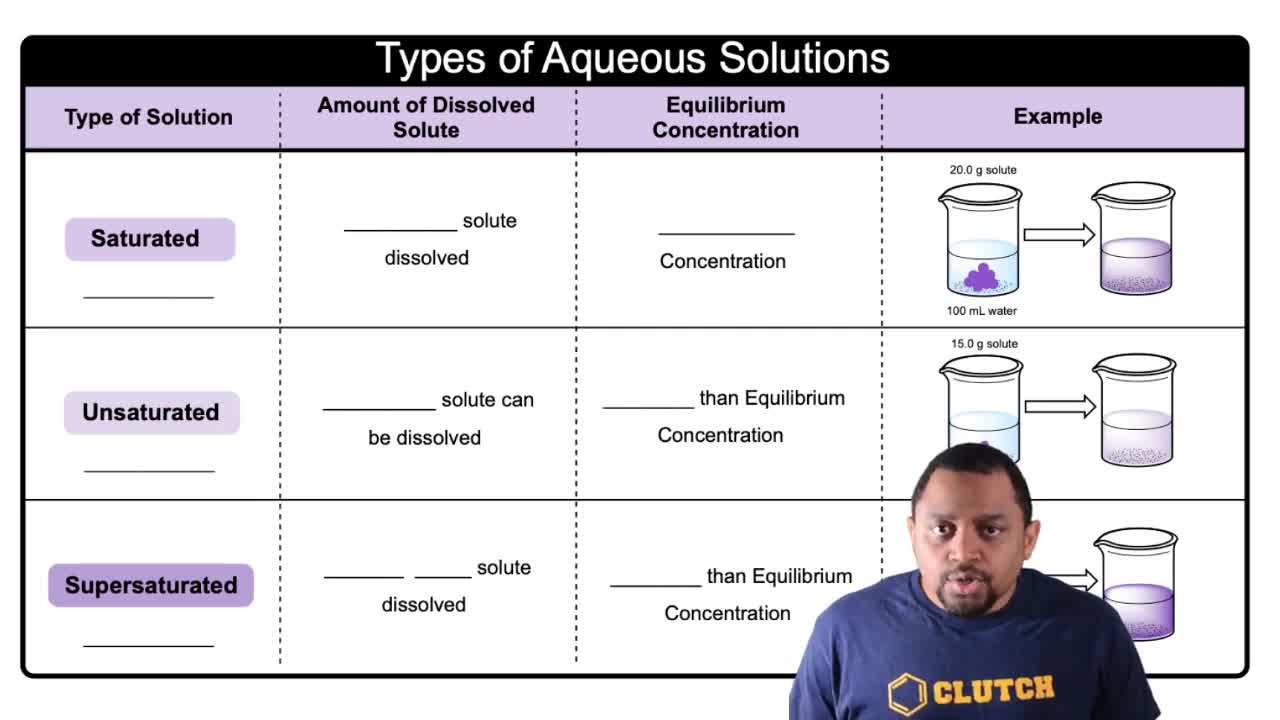
Types of Aqueous Solutions
14. Solutions
Types of Aqueous Solutions
Additional 2 creators.
Learn with other creators
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
The solubility of KClO3 in water at 30ºC is 10 g per 100 mL of water. A 0.95 M solution of KClO3 in water at 30ºC is:
3073views8rank2comments - Multiple ChoiceAt 88 °C the solubility of KNO3 is 88 g per 100 g of water. Would a solution that has a molarity of 0.990 M KNO3 and a density of 0.988 g/mL be considered unsaturated, saturated, or supersaturated?1036views
- Open Question
If more powdered kool-aid is added to the same amount of water, what happens to the solution?
1048views - Open Question
One way to determine the degree of saturation of a solid-liquid solution is to drop a crystal
829views - Open Question
A solution that contains less solute than a saturated solution at a given temperature and pressure.
937views - Open Question
The particles of matter that are dissolved in a solution are known as what?
811views - Multiple ChoiceWhich of the following types of aqueous solutions is formed when salt (such as NaCl) is dissolved in water?45views
- Multiple ChoiceWhich of the following substances forms a homogeneous mixture when mixed with water?58views