12. Molecular Shapes & Valence Bond Theory
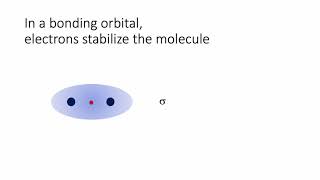
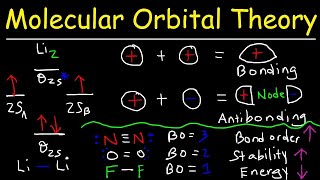
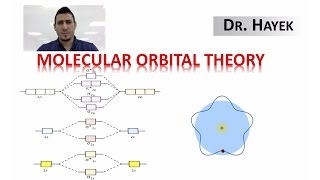
MO Theory: Bond Order
12. Molecular Shapes & Valence Bond Theory
MO Theory: Bond Order
Additional 2 creators.
Learn with other creators
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
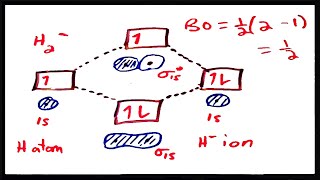
Apply Molecular Orbital Theory to determine the bond order of HHe+ ion.
1363views5rank1comments - Multiple Choice
Apply molecular orbital theory to predict which species has the strongest bond.
a) O2 b) O2– c) O2+ d) All the bonds are equivalent
2200views3rank3comments - Multiple Choice
Using Molecular Orbital Theory, answer the following questions dealing with carbon mononitride, CN.
1914views2rank2comments - Multiple ChoiceWhich of the following is not consistent with the valence bond theory description of the formation of a chemical bond?707views
- Open Question
Determine the bond order in a molecule or ion with 12 valence electrons.
665views - Open QuestionUse the molecular orbital diagram shown to determine which of the following is most stable.1678views
- Open Question
Complete the molecular orbital diagram for CN−. Note that the 1𝑠 orbitals are not shown.
1105views