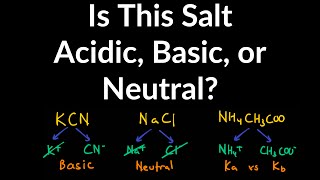
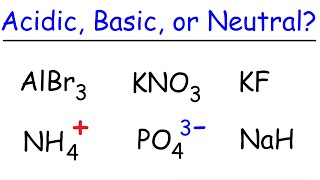
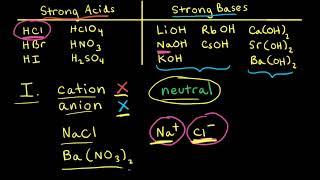
17. Acid and Base Equilibrium
Ionic Salts
17. Acid and Base Equilibrium
Ionic Salts
Showing 8 of 8 videos
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
Determine if each of the following compounds will create an acidic, basic or neutral solution.
a) LiC2H3O2 b) C6H5NH3Br
2100views2comments - Multiple Choice
Determine if each of the following compounds will create an acidic, basic or neutral solution.
a) Co(HSO4)2 b) Sr(HSO3)2
1888views3comments - Multiple Choice
Determine if each of the following compounds will create an acidic, basic or neutral solution.
a. C3H7NH3F
1086views2rank5comments - Multiple Choice
Determine the pH of a 0.55 M NaCN solution. The Ka of hydrocyanic acid, HCN, is 4.9 x 10-10.
2582views1rank11comments - Open QuestionClassify each of the following anions as basic or neutral.723views
- Open QuestionArrange the following solutions in order of increasing acidity:895views
- Open Question
For Mn3+, write an equation that shows how the cation acts as an acid.
557views