Insulin is synthesized as preproinsulin, which has 81 amino acids. How many heterocyclic bases must be present in the informational DNA strand to code for preproinsulin (assuming no introns are present)?
Draw the structures of adenine and uracil (which replaces thymine in RNA), and show the hydrogen bonding that occurs between them.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Nucleotide Structure
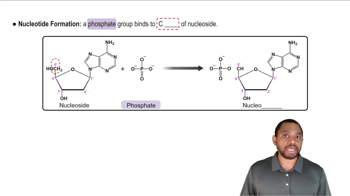
Hydrogen Bonding

Base Pairing Rules
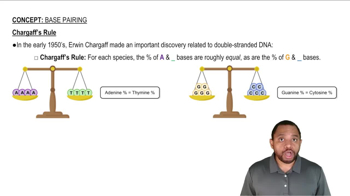
Suppose that 22% of the nucleotides of a DNA molecule are deoxyadenosine and during replication the relative amounts of available deoxynucleoside triphosphates are 22% dATP, 22% dCTP, 28% dGTP, and 28% dTTP. What deoxynucleoside triphosphate is limiting to the replication? Explain.
Write the complementary sequence of bases for each DNA strand shown next.
a. 5′T-A-T-A-C-T-G 3′
(a) DNA and RNA, like proteins, can be denatured to produce unfolded or uncoiled strands. Heating DNA to what is referred to as its “melting temperature” denatures it (the two strands of the double helix become separated). Why does a longer strand of DNA have a higher melting temperature than a shorter one?
a. What is meant by the term base pairing?
What does it mean to speak of bases as being complementary?
