Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic Compounds
Ionic compounds are formed when positively charged ions (cations) and negatively charged ions (anions) bond together through electrostatic forces. The overall charge of the compound must be neutral, meaning the total positive charge must balance the total negative charge. Understanding how these charges interact is essential for writing correct ionic formulas.
Recommended video:
Charge Balance
Charge balance is a fundamental principle in forming ionic compounds. Each ion carries a specific charge, and when combining ions, the total positive charge from cations must equal the total negative charge from anions. For example, in the case of Ca²⁺ (which has a +2 charge) and S²⁻ (which has a -2 charge), one of each ion combines to create a neutral compound.
Recommended video:
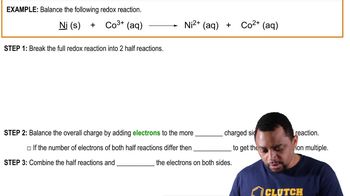
Balancing Redox Reactions (Simplified) Example 2
Chemical Formula Representation
The chemical formula of an ionic compound represents the ratio of ions in the compound. It is typically written with the cation first followed by the anion. For the compound formed from Ca²⁺ and S²⁻, the formula is CaS, indicating a 1:1 ratio of calcium ions to sulfide ions, which reflects the balance of charges.
Recommended video:
Molecular Representations Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:46m
1:46m