Here are the essential concepts you must grasp in order to answer the question correctly.
Gene Expression and Alternative Splicing
Gene expression refers to the process by which information from a gene is used to synthesize functional gene products, typically proteins. Alternative splicing is a mechanism that allows a single gene to produce multiple protein isoforms by including or excluding certain sequences during RNA processing. This can lead to a situation where fewer protein-coding genes can generate a much larger variety of proteins, contributing to the observed discrepancy.
Recommended video:
Penetrance and Expressivity
Post-Translational Modifications
Post-translational modifications (PTMs) are chemical changes that occur to proteins after their synthesis, which can significantly alter their function, activity, and stability. These modifications, such as phosphorylation, glycosylation, and ubiquitination, can create diverse protein forms from a single polypeptide chain, further increasing the number of functional proteins beyond the number of genes.
Recommended video:
Post Translational Modifications
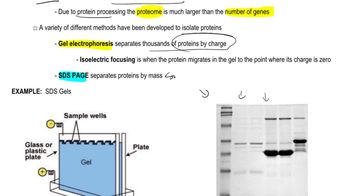
Proteomics and Protein Diversity
Proteomics is the large-scale study of proteins, particularly their functions and structures. It encompasses techniques that analyze the entire protein complement of a cell or organism, revealing the complexity and diversity of proteins produced. The ability of human cells to synthesize over 100,000 different proteins highlights the intricate regulatory mechanisms and interactions that contribute to protein diversity, which can exceed the number of genes.
Recommended video:
 Verified step by step guidance
Verified step by step guidance