Here are the essential concepts you must grasp in order to answer the question correctly.
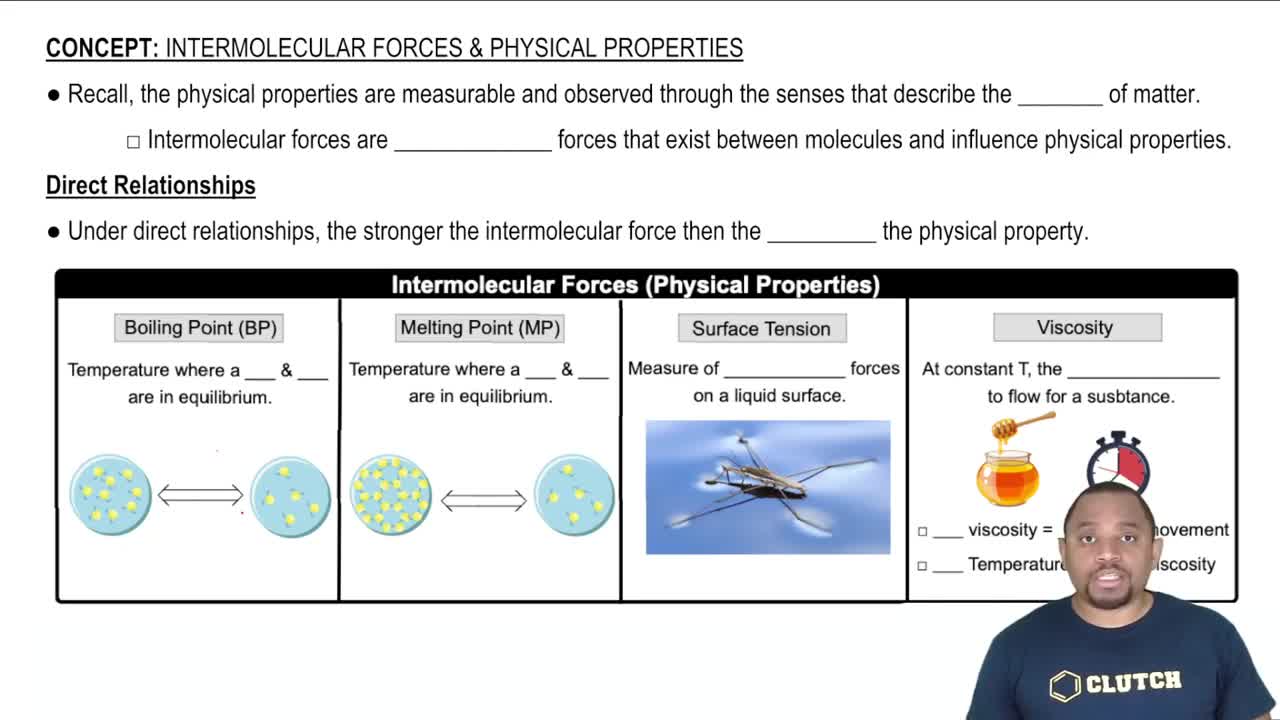
Viscosity
Viscosity is a measure of a fluid's resistance to flow, influenced by intermolecular forces and the size and shape of its molecules. Higher viscosity indicates a thicker fluid that flows more slowly, while lower viscosity corresponds to a thinner fluid that flows more easily. In the context of water and dimethyl sulfide, the differences in viscosity can be attributed to their molecular interactions and structural characteristics.
Recommended video:
Intermolecular Forces and Properties
Intermolecular Forces
Intermolecular forces are the attractive forces between molecules that influence physical properties like boiling point, melting point, and viscosity. Water exhibits strong hydrogen bonding due to its polar nature, leading to higher viscosity. In contrast, dimethyl sulfide has weaker van der Waals forces, resulting in lower viscosity as its molecules can move past each other more easily.
Recommended video:
Intermolecular vs Intramolecular Forces
Molecular Structure
The molecular structure of a substance, including its shape and the presence of functional groups, significantly affects its physical properties. Water's bent shape and polar O-H bonds create a highly cohesive liquid, while dimethyl sulfide's linear structure and sulfur atom lead to less cohesive interactions. This structural difference contributes to the observed disparity in viscosity between the two substances.
Recommended video: