Here are the essential concepts you must grasp in order to answer the question correctly.
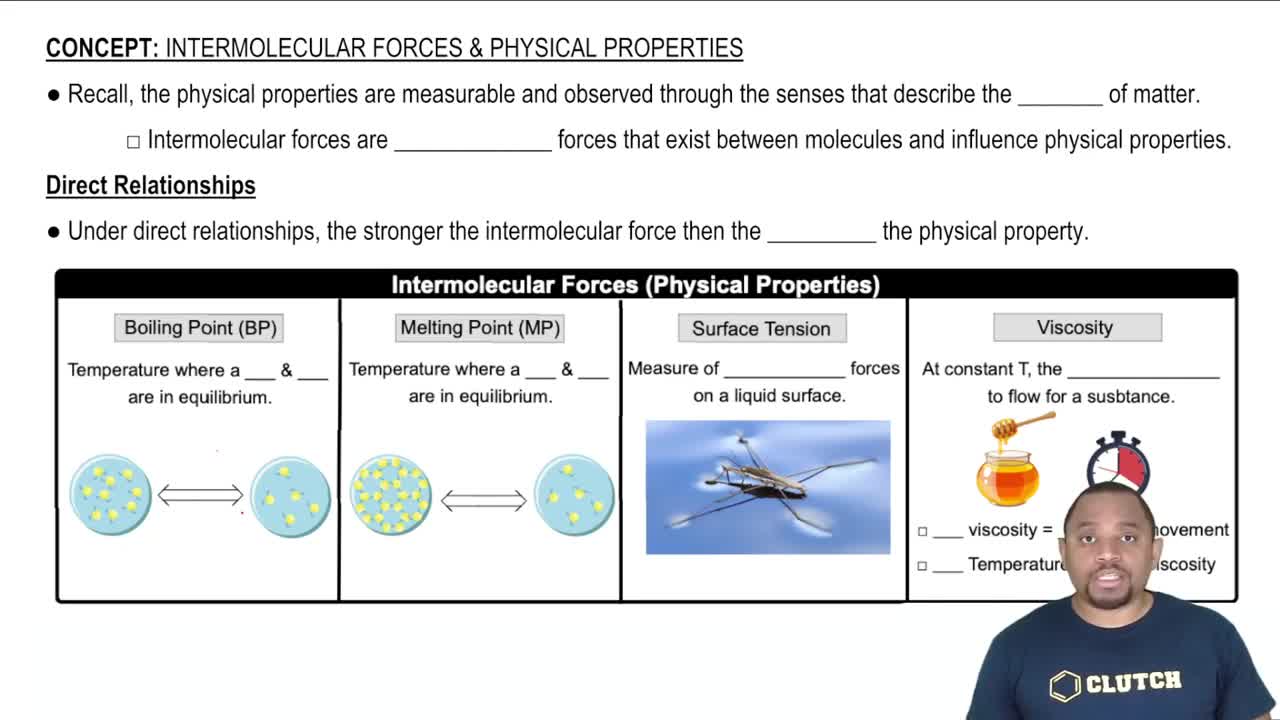
Viscosity
Viscosity is a measure of a fluid's resistance to flow. It reflects how easily molecules within a substance can move past one another. Higher viscosity indicates a thicker fluid that flows more slowly, while lower viscosity corresponds to a thinner fluid that flows more easily. Factors influencing viscosity include temperature, molecular size, and intermolecular forces.
Recommended video:
Intermolecular Forces and Properties
Intermolecular Forces
Intermolecular forces are the attractive forces between molecules that influence physical properties such as boiling point, melting point, and viscosity. These forces include hydrogen bonding, dipole-dipole interactions, and London dispersion forces. Substances with stronger intermolecular forces typically exhibit higher viscosity due to the increased energy required to overcome these attractions during flow.
Recommended video:
Intermolecular vs Intramolecular Forces
Hydroxyl Group
The hydroxyl group (-OH) is a functional group found in alcohols, such as 1-hexanol. This group is capable of forming hydrogen bonds, which significantly increases the viscosity of the substance compared to hydrocarbons like hexane that lack such polar functional groups. The presence of the hydroxyl group enhances intermolecular interactions, leading to a thicker, more viscous liquid.
Recommended video: