Here are the essential concepts you must grasp in order to answer the question correctly.
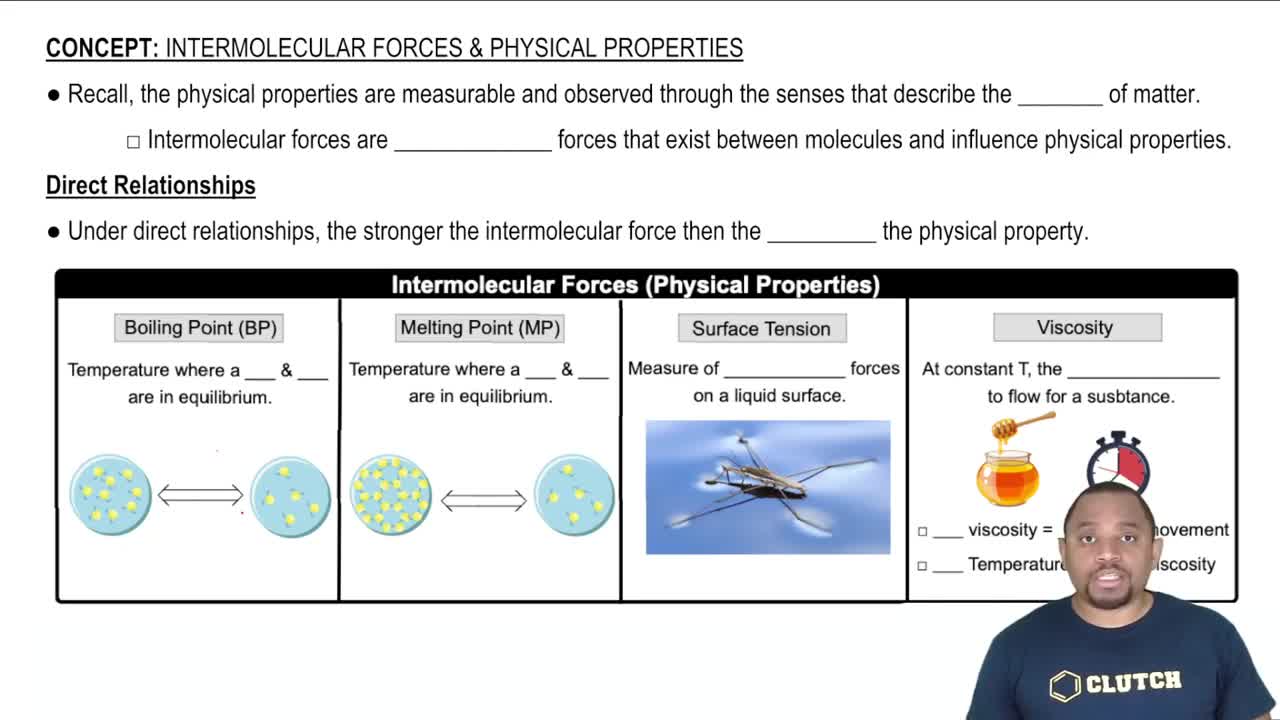
Surface Tension
Surface tension is a physical property of liquids that describes the elastic-like force at the surface, which makes it behave as if it were covered by a stretched elastic membrane. It arises from the cohesive forces between liquid molecules, which are stronger at the surface due to the imbalance of intermolecular forces. Higher surface tension indicates a greater ability of a liquid to resist external forces.
Recommended video:
Intermolecular Forces and Properties
Intermolecular Forces
Intermolecular forces are the forces of attraction or repulsion between neighboring particles (atoms, molecules, or ions). These forces include hydrogen bonding, dipole-dipole interactions, and London dispersion forces. The strength and type of these forces significantly influence a substance's physical properties, including surface tension, with stronger intermolecular forces typically leading to higher surface tension.
Recommended video:
Intermolecular vs Intramolecular Forces
Molecular Structure and Polarity
The molecular structure and polarity of a substance play a crucial role in determining its surface tension. Polar molecules, which have a significant difference in electronegativity between atoms, tend to have stronger intermolecular forces due to dipole interactions. In contrast, nonpolar molecules exhibit weaker London dispersion forces. Understanding the polarity and structure of CCl4 and CH2Br2 is essential for predicting which has higher surface tension.
Recommended video: