Textbook Question
Calculate the uncertainty in the position of (a) an electron moving at a speed of 13.00 { 0.012 * 105 m/s
900
views
1
rank



Calculate the uncertainty in the position of (a) an electron moving at a speed of 13.00 { 0.012 * 105 m/s
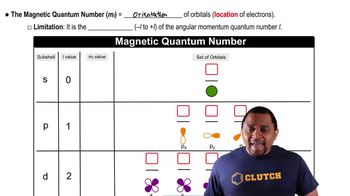
(a) For n = 4, what are the possible values of l?
How many unique combinations of the quantum numbers l and ml are there when (b) n = 5?
Give the numerical values of n and l corresponding to each of the following orbital designations: (d) 5d.
Give the values for n, l, and ml for (a) each orbital in the 3p subshell.
A certain orbital of the hydrogen atom has n = 4 and l = 3. (a) What are the possible values of ml for this orbital?