 Back
BackProblem 1a
The speed of sound in dry air at 20 °C is 343 m/s and the lowest frequency sound wave that the human ear can detect is approximately 20 Hz. (a) What is the wavelength of such a sound wave?
Problem 1b
The speed of sound in dry air at 20 °C is 343 m/s and the lowest frequency sound wave that the human ear can detect is approximately 20 Hz. (b) What would be the frequency of electromagnetic radiation with the same wavelength?
Problem 2a
A popular kitchen appliance produces electromagnetic radiation with a frequency of 2450 MHz. With reference to Figure 6.4, answer the following: (a) Estimate the wavelength of this radiation.
Problem 2b
A popular kitchen appliance produces electromagnetic radiation with a frequency of 2450 MHz. With reference to Figure 6.4, answer the following: (b) Would the radiation produced by the appliance be visible to the human eye?

Problem 2c
A popular kitchen appliance produces electromagnetic radiation with a frequency of 2450 MHz. With reference to Figure 6.4, answer the following: (c) If the radiation is not visible, do photons of this radiation have more or less energy than photons of visible light?

Problem 2d
A popular kitchen appliance produces electromagnetic radiation with a frequency of 2450 MHz. With reference to Figure 6.4, answer the following: (d) Which of the following is the appliance likely to be? (i) A toaster oven, (ii) A microwave oven, or (iii) An electric hotplate.
Problem 4a
Stars do not all have the same temperature. The color of light emitted by stars is characteristic of the light emitted by hot objects. Telescopic photos of three stars are shown below: (i) the Sun, which is classified as a yellow star, (ii) Rigel, in the constellation Orion, which is classified as a blue-white star, and (iii) Betelgeuse, also in Orion, which is classified as a red star. (a) Place these three stars in order of increasing temperature. (i) sun (ii) Rigel (iii) Betelguese
Problem 5a
The familiar phenomenon of a rainbow results from the diffraction of sunlight through raindrops. (a) Does the wavelength of light increase or decrease as we proceed outward from the innermost band of the rainbow?
Problem 6b
A certain quantum-mechanical system has the energy levels shown in the accompanying diagram. The energy levels are indexed by a single quantum number n that is an integer. (b) Which quantum numbers are involved in the transition that requires the least energy?

Problem 7a
Consider the three electronic transitions in a hydrogen atom shown here, labeled A, B, and C. (a) Three electromagnetic waves, all drawn on the same scale, are also shown. Each corresponds to one of the transitions. Which electromagnetic wave (i), (ii), or (iii), is associated with electronic transition C?

Problem 7b3
Consider the three electronic transitions in a hydrogen atom shown here, labeled A, B, and C. (b) Calculate the energy of the photon emitted for each transition.

Calculate the energy of the photon emitted for transition C.
Problem 7c2
Consider the three electronic transitions in a hydrogen atom shown here, labeled A, B, and C. (c) Calculate the wavelength of the photon emitted for each transition. Do any of these transitions lead to the emission of visible light? If so which one(s)?

Calculate the wavelength of the photon emitted for transition B.
Problem 8b
Consider a fictitious one-dimensional system with one electron. The wave function for the electron, drawn below, is c1x2 = sin x from x = 0 to x = 2p. (b) At what value or values of x will there be the greatest probability of finding the electron?
Problem 9a
The contour representation of one of the orbitals for the n = 3 shell of a hydrogen atom is shown here. (a) What is the quantum number l for this orbital?

Problem 9c
The contour representation of one of the orbitals for the n = 3 shell of a hydrogen atom is shown here. (c) In which of the following ways would you modify this sketch if the value of the magnetic quantum number, ml, were to change? (i) It would be drawn larger, (ii) the number of lobes would change, (iii) the lobes of the orbital would point in a different direction, (iv) there would be no change in the sketch.
Problem 10b
The accompanying drawing shows a contour plot for a dyz orbital. Consider the quantum numbers that could potentially correspond to this orbital. (b) What is the value of the angular momentum quantum number, l?
Problem 10c
The accompanying drawing shows a contour plot for a dyz orbital. Consider the quantum numbers that could potentially correspond to this orbital. (c) What is the largest possible value of the magnetic quantum number, ml?
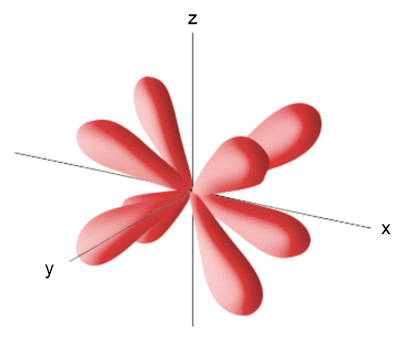
Problem 10d
The accompanying drawing shows a contour plot for a dyz orbital. Consider the quantum numbers that could potentially correspond to this orbital. (d) The probability density goes to zero along which of the following planes: xy, xz, or yz?

Problem 11a
Four possible electron configurations for a carbon atom are shown below, but only one schematic represents the correct configuration for a carbon atom in its ground state. Which one is the correct electron configuration?

Problem 11b
Four possible electron configurations for a nitrogen atom are shown below, but only one schematic represents the correct configuration for a nitrogen atom in its ground state. Which configurations violate the Pauli exclusion principle?
Problem 12
State where in the periodic table these elements appear: (a) elements with the valence-shell electron configuration ns2np5 (b) elements that have three unpaired p electrons (c) an element whose valence electrons are 4s24p1 (d) the d-block elements [Section 6.9]
- The wavenumber l is the number of waves that exist over a specified distance, very often 1 cm. The wavenumber can easily be calculated by taking the reciprocal of the wavelength. Give typical wavenumbers for (a) X-rays (λ = 1 nm) (b) visible light (λ = 500 nm) (c) microwaves (λ = 1 mm).
Problem 13
- Carbon dioxide in the atmosphere absorbs energy in the 4.0–4.5 mm range of the spectrum. (a) Calculate the frequency of the 4.0 mm radiation.
Problem 14
Problem 15a
Label each of the following statements as true or false. For those that are false, correct the statement. (a) Visible light is a form of electromagnetic radiation.
Problem 15b
Label each of the following statements as true or false. For those that are false, correct the statement. (b) Ultraviolet light has longer wavelengths than visible light.
Problem 15c
Label each of the following statements as true or false. For those that are false, correct the statement. (c) X rays travel faster than microwaves.
Problem 16
Determine which of the following statements are false and correct them. Determine which of the following statements are false and correct them. (a) The frequency of radiation increases as the wavelength increases. (b) Electromagnetic radiation travels through a vacuum at a constant speed, regardless of wavelength. (c) Infrared light has higher frequencies than visible light. (d) The glow from a fireplace, the energy within a microwave oven, and a foghorn blast are all forms of electromagnetic radiation.
Problem 17
Arrange the following kinds of electromagnetic radiation in order of increasing wavelength: infrared, green light, red light, radio waves, X rays, ultraviolet light.
- List the following types of electromagnetic radiation in order of increasing wavelength: (a) the gamma rays produced by a radioactive nuclide used in medical imaging; (b) radiation from an FM radio station at 93.1 MHz on the dial; (c) a radio signal from an AM radio station at 680 kHz on the dial; (d) the yellow light from sodium vapor streetlights; (e) the red light of a light-emitting diode, such as in a calculator display.
Problem 18
Problem 19a
(a) What is the frequency of radiation that has a wavelength of 10 µm, about the size of a bacterium?
