The accompanying drawing shows a contour plot for a dyz orbital. Consider the quantum numbers that could potentially correspond to this orbital. (d) The probability density goes to zero along which of the following planes: xy, xz, or yz?
Ch.6 - Electronic Structure of Atoms
Chapter 6, Problem 12
State where in the periodic table these elements appear: (a) elements with the valence-shell electron configuration ns2np5 (b) elements that have three unpaired p electrons (c) an element whose valence electrons are 4s24p1 (d) the d-block elements [Section 6.9]
 Verified step by step guidance
Verified step by step guidance1
Identify the general electron configuration for each case and relate it to the periodic table sections: s-block, p-block, d-block, and f-block.
For part (a), recognize that the electron configuration ns2np5 corresponds to halogens, which are located in Group 17 of the p-block.
For part (b), elements with three unpaired p electrons typically have a p3 configuration, placing them in Group 15 of the p-block.
For part (c), the electron configuration 4s24p1 indicates an element in the 4th period of the p-block, specifically in Group 13.
For part (d), understand that the d-block elements are transition metals, which are found in the central block of the periodic table, specifically Groups 3 through 12.
Recommended similar problem, with video answer:

Verified Solution
This video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Valence Electron Configuration
Valence electron configuration describes the distribution of electrons in the outermost shell of an atom. The notation 'ns2np5' indicates that the element has two electrons in the s subshell and five in the p subshell of its highest energy level, which corresponds to the halogens in Group 17 of the periodic table.
Recommended video:
Guided course

Transition Metals Valence Electrons
Unpaired Electrons
Unpaired electrons are those that occupy an orbital alone rather than in pairs. Elements with three unpaired p electrons typically belong to Group 15 of the periodic table, where the p subshell has three electrons, leading to unique chemical properties and reactivity.
Recommended video:
Guided course

Electron Geometry
d-block Elements
The d-block elements, also known as transition metals, are found in Groups 3 to 12 of the periodic table. These elements are characterized by the filling of d orbitals and exhibit properties such as variable oxidation states and the ability to form colored compounds, making them essential in various chemical reactions and applications.
Recommended video:
Guided course
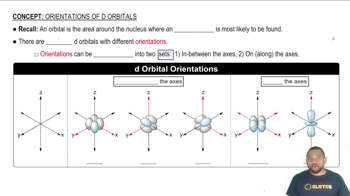
d Orbital Orientations
Related Practice
Textbook Question
737
views
Textbook Question
Four possible electron configurations for a carbon atom are shown below, but only one schematic represents the correct configuration for a carbon atom in its ground state. Which one is the correct electron configuration?
2654
views
1
comments
Textbook Question
Four possible electron configurations for a nitrogen atom are shown below, but only one schematic represents the correct configuration for a nitrogen atom in its ground state. Which configurations violate the Pauli exclusion principle?
751
views
Open Question
The wavenumber l is the number of waves that exist over a specified distance, very often 1 cm. The wavenumber can easily be calculated by taking the reciprocal of the wavelength. Give typical wavenumbers for (a) X-rays (λ = 1 nm) (b) visible light (λ = 500 nm) (c) microwaves (λ = 1 mm).
Open Question
Carbon dioxide in the atmosphere absorbs energy in the 4.0–4.5 mm range of the spectrum. (a) Calculate the frequency of the 4.0 mm radiation.
Textbook Question
Label each of the following statements as true or false. For those that are false, correct the statement. (a) Visible light is a form of electromagnetic radiation.
923
views
