Textbook Question
How many unique combinations of the quantum numbers l and ml are there when (b) n = 5?
1196
views
 Verified step by step guidance
Verified step by step guidance


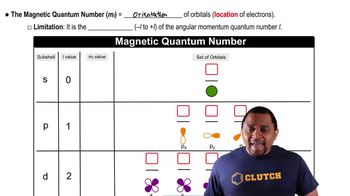
How many unique combinations of the quantum numbers l and ml are there when (b) n = 5?
Give the numerical values of n and l corresponding to each of the following orbital designations: (a) 3p.
Give the numerical values of n and l corresponding to each of the following orbital designations: (d) 5d.
A certain orbital of the hydrogen atom has n = 4 and l = 3. (a) What are the possible values of ml for this orbital?
A certain orbital of the hydrogen atom has n = 4 and l = 3. (b) What are the possible values of ms for the orbital?
Which of the following represent impossible combinations of n and l? (a) 1p (b) 4s (c) 5f (d) 2d