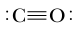Formal charge is a concept used to assign a charge to atoms in a molecule, based on the assumption that electrons are shared equally between bonded atoms, regardless of their electronegativity. In reality, more electronegative elements tend to attract electrons more strongly, leading to an uneven distribution of electron density. However, for the purpose of calculating formal charge, this unequal sharing is not considered.
When discussing formal charge, it is essential to differentiate between bonding electrons and nonbonding electrons. Bonding electrons are those involved in forming bonds with other atoms, while nonbonding electrons are those that do not participate in bonding.
The formula for calculating formal charge is as follows:
\[ \text{Formal Charge} = \text{Valence Electrons} - \text{Bonds} + \text{Nonbonding Electrons} \]
In this formula, the valence electrons correspond to the group number of the element in the periodic table. When counting nonbonding electrons, each electron is counted individually. By calculating the formal charge for each atom in a molecule, one can determine the net charge, which is the sum of all individual formal charges within that compound.
Understanding formal charge is crucial for predicting the stability and reactivity of molecules, as it provides insight into the distribution of electrons and the overall charge of the compound.