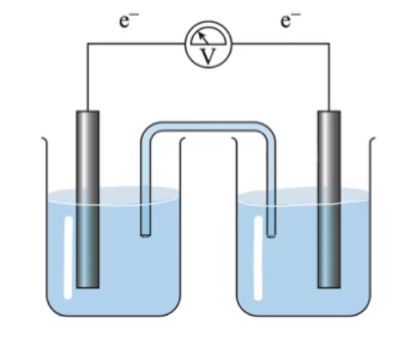
A galvanic cell, also known as a voltaic cell, is a device that converts chemical energy into electrical energy through spontaneous redox reactions. The major components of a galvanic cell include the anode, cathode, salt bridge, and voltmeter, each playing a crucial role in the cell's operation.
The anode is the negatively charged electrode where oxidation occurs, meaning it is the site where electrons are lost. In a typical galvanic cell, the anode is often made of a metal such as zinc. During the oxidation process, zinc atoms lose electrons and enter the solution as zinc ions:
Zn (s) → Zn2+ (aq) + 2e-
Conversely, the cathode is the positively charged electrode where reduction takes place, which involves the gain of electrons. In our example, the cathode is typically made of copper. Here, copper ions in the solution gain electrons and are reduced to solid copper:
Cu2+ (aq) + 2e- → Cu (s)
Connecting the anode and cathode is the salt bridge, a crucial component that allows for the flow of neutral ions between the two half-cells. This bridge helps to maintain electrical neutrality by balancing the charge buildup that occurs as the redox reactions proceed. Neutral ions, such as sodium (Na+), potassium (K+), and bromide (Br-), flow through the salt bridge to counteract the increasing concentration of cations in the anode compartment and anions in the cathode compartment.
Finally, the voltmeter is an essential instrument that measures the voltage produced by the galvanic cell. As electrons flow from the anode to the cathode through an external circuit, the voltmeter records the electrical potential difference, providing a quantitative measure of the electricity generated. The voltage (V) is a key indicator of the cell's efficiency and the extent of the chemical reactions occurring within the cell.
In summary, a galvanic cell operates through the oxidation of the anode and the reduction of the cathode, facilitated by the salt bridge and measured by the voltmeter, effectively converting chemical energy into electrical energy.