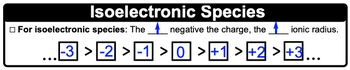
For a majority of the periodic trends, looking at the periodic table can tell you if they're increasing or decreasing. In all our periodic trends, we're heading towards the top right corner and heading in that directional either increase or decrease that particular periodic trend. Now ionic radius doesn't fit into this idea. Ionic radius does not involve looking at the periodic table or Let's say ionic radius equals the distance between an ion's nucleus and its outer shell, and the periodic trend is this. The ionic radius increases as the number of electrons of that ion increases.
So we're going to say here when we're looking at ionic radius, we're looking at ions and ions can either be positive or negative. For positive ions, which are cations, we're going to say cations tend to be smaller than their neutral parent form. So what do I mean by this? Well, if we take a look here at lithium, the lithium atom is I1S2S1. It has one electron in its valence electron shell, right? Because its second shell only has one. When I become lithium ion I lose 1 electron, I lose it from that valence shell. So now the lithium ion no longer has 2 shells, it only has one shell that contains electrons and we can see that there is a drop in the size for that particular ion. So just remember the neutral form of the element is bigger than the cation form. So the general trend is losing an electron or electrons causes a decrease in your ionic radius.
Now if we're looking at the anions, anions tend to be larger than their neutral parent forms. Oxygen is S1S2S2P4 here. These electrons are in the second shell, and there's six of them total. When we become the oxide ion, we gain two more electrons. So now my outer shell has eight electrons. It may not be as apparent, but the anion is slightly bigger than the neutral form because Y we said earlier the ionic radius increases as the number of electrons increases. So basically more electrons equal larger ionic radius.
So we're going to say gaining an electron or electrons causes an increase in your ionic radius. So just remember we don't look at the periodic table to determine the trend in ionic radius. Instead, we look at the total number of electrons that ion has. The more electrons it has, the bigger its ionic radius.