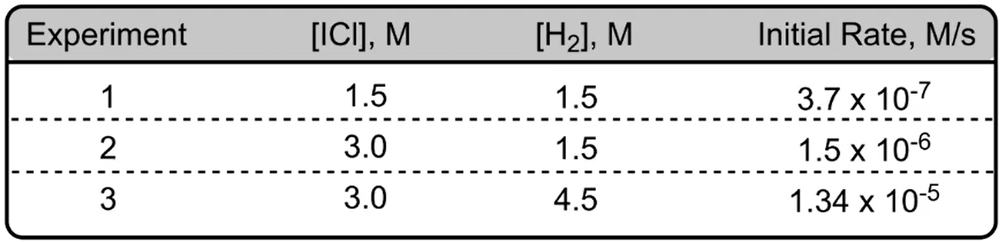
The initial rates of reaction 2 NO gas + Cl2 gas giving us 2 NOCl gas are given below. Here we have experiments 1-2 and 3 and each experiment has attached to it reacting concentrations and an initial rate. Now here it says determine the new rate if given new initial concentrations for NO and Cl2. Now remember our sequence for solving is we first have to determine our reaction orders. Once we do that, then we can figure out our rate constant K, and then finally we can determine our new rate.
All right. So for step one, we're going to choose a reactant and look at two experiments where its concentration changes, but the other reactants stay the same. These other reactants we're going to ignore. So you're going to ignore the reactants whose concentrations remain the same. Here, we're going to look for the reaction order of NO. So let's make it X, Cl2 would have Y. You can make the many variables you want, A, B, C, whatever. Here I just do X & Y. We have to look for two experiments where NO is changing but Cl2 is staying the same. That occurs with experiments 2 and 3. With experiments 2 and 3, we see NO is changing and the concentrations of Cl2 remain the same.
Once we've figured that out, we go to Step 2 where we create a pair of ratios for the reactant that sets rates equal to concentrations of the reactants. This is important. You place the larger rate value on top of the smaller rate value to get whole numbers when solving. And here you're going to raise the reactant concentrations to an unknown power or variable for the reaction order in salt. Alright, so if we take a look here, experiments 2 and 3, we can see that rate 3, its rate initial rate 3. Because it's connected to experiment 3, it's larger than initial rate 2. So that would mean that we're going to have rate 3 over rate 2 equals the concentrations of NO to the X. Now we're going to plug in the number so
18.2
9.08
=
0.0500
0.0250
(
2
)
X
18.2 / 9.08 is approximately 2 and then 0.0500 / 0.0250 is 2 and it's still 2X. Now you say 2 to what number gives you 2? The answer would be 1. So the reaction order for NO is 1.
Now we're going to do the same thing for step three. We're going to repeat the process for any remaining reactants until all reaction orders are determined, right? So now we have to look for Cl2. We look for two experiments where Cl2 is changing, but NO is staying the same. We see that happen with experiments 1 and 2. Now here initial rate 1 is larger than initial rate 2. So if we come down here, we're going to say rate 1 over rate 2 equals Cl2 divided by Cl2 to the Y. So let's see. That was 18.2 / 9.08. And then here we're going to plug in the concentrations for Cl2. So here for Cl2 it's 0.0510 and this is 0.0255 to the Y. This also comes out to 2 = 2Y, 2 to one number gives us 2. The answer is 1. So it's first order for both of our reactants.
Now Step 4, if necessary. Here it is necessary because we need to find our new rate, and to find our new rate we have to figure out our rate constant. So here we're going to say, if necessary to solve for the rate constant K, plug in the reactant concentration and react in orders into the rate law. All right. So here we're going to say that our rate, we're going to say rate 1. You can use any of the rates, but just to keep it simple, always go with rate 1. So rate 1 equals K and we're going to say here NO to the one, Cl2 to the one, OK. And then we're going to start plugging the numbers. So 18.2, we're looking for K. We don't know what K is. Come back up here. Let's use a concentration for. So since we're using rate 1, we have to use the concentrations of experiment 1. So that's 0.0250 and 0.0510 to the one and 0.0510 to the one. Alright, so here when we multiply these together, that's going to give me 0.001275 still multiplying with K. Rate 1 again is 18.2. Divide both sides by 0.001275 and we'll have our K. Here K equals 14117.647.
Now here we could technically give the units for K because remember to find the units of K it's M to the negative N + 1 times time inverse. And here's our overall order, which is 2, and it comes from adding your reaction orders together, so that's -2 + 1. Here. Let's just use seconds inverse, since our initial rates are molarities per second, so that would be M to the -1 times seconds inverse. OK, finally, we can figure out our new rate and again if necessary, we're going to say to solve for the new rate, plug in the K, the reaction orders and the additional reaction concentrations given. So here we're going to say that our new rate equals K and we're going to plug in the new concentrations given to us in the very beginning. So let's come up here. So here we have 0.0730 for our NO and then we have 0.0510 for our Cl2. So those are the new concentrations we're going to plug into here. So let's come down here. So this is 0.0730 actually and that's again for NO to the one and Cl2 to the one. So new rate. So K we got is 14117.647, NO is 0.0730 to the one, Cl2 is 0.0510 to the one. When we plug all that in, that gives us 52.6 molarities per second for our new rate.
So we can see that if they're asking us for the new rate it is quite an ordeal. You have to go through a lot of things to get to your final answer. Remember, the sequence we're solving is important. First, figure out reaction orders before you can figure out your rate constant K before you can figure out your final new rate.