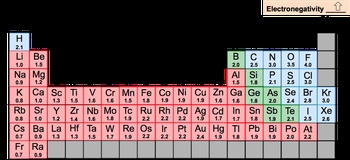
Electronegativity, abbreviated as EN, is a measurement of an element's ability to attract electrons to itself. Oftentimes it's confused with electron affinity. Electron affinity is different. Electron affinity is the energy released when an electron is added to an element. So remember the distinction here.
In 1932, the American chemist Linus Pauling proposed electronegativity values for the elements. Now this is pretty great. Electronegativity is one of my favorite topics in Gen. Chem, and there's not that many American chemists credited with some of these fundamental concepts. Usually it's people from Russia or Germany or some other country. So it's great to see an American chemist in the form of Linus Pauling getting credit for such an important idea.
Now we're going to say here the periodic trend is electronegativity increases as we move from left to right across a period and going up a group. So as we're heading to this top right corner of the periodic table, electronegativity will increase. Now there of course are exceptions in chem. Those exist with the transition metals because of variations in their D&F orbitals. With these D&F orbitals, there's expansions that happen in shells and this kind of messes up the values of electronegativity.
You'll also notice here that some of the noble gases don't have electronegativity values and that's because they don't want to attract electrons to themselves. We're also going to notice that a lot of the bottom row is blank as well because they're so large, so heavy, they're too unstable to talk about electronegativity. Now in college, what are we all looking to get? That perfect 4.0 GPA.
So fluorine is the most electronegative element on the periodic table. So this is incredibly important to always remember. It is the most electronegative and then francium is the least electronegative element on the periodic table. So just remember, when we talk about electronegativity, it's us attracting electrons to ourselves. When the electron actually adds onto the gaseous state of an element, then that becomes electron affinity.