With the spin quantum number we actually look at the individual spins of the electrons within a given orbital. We're going to say an orbital can hold a maximum of two electrons that have opposite spins according to the Pauli exclusion principle. Now the Pauli exclusion principle says that no two electrons within the same orbital can have the same four quantum numbers by having opposite spins. That affects their spin quantum number, which is Ms.
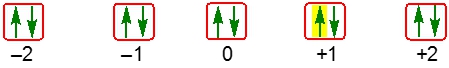
Now it deals with the rotational spin of an electron inside an atomic orbital. We're going to say here that the way we do it is we start out filling an orbital with an electron that points up followed by the next one pointing down. This indicates that each electron has an opposite spin. We're going to say an electron that points up has an Ms value of 12, and one that points down has an Ms value of -12.
So here we have our illustration of an orbital in red, and we have those two electrons within that orbital. One is spinning up, the first one, and then one is spinning down. So just remember when it comes to the spin of an electron within an orbital, we look at the Ms quantum number.