Now, the words temperature and heat are often used interchangeably with one another, but they're not the same thing. They represent different aspects of thermal energy. Now thermal energy itself is the sum of all kinetic and potential energies of all atoms in an object. Now when we say kinetic energy, we know that kinetic energy is the energy of motion. So the movement of molecules, the movement of atoms, potential energy is just the energy of position. Were those molecules are related? Are they 100 feet off the ground? Are they 10 feet off the ground? Where exactly are they located?
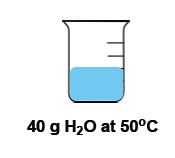
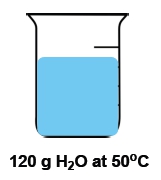
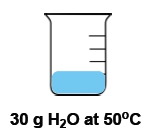
Now temperature and heat again are used interchangeably with one another, but they're not the same thing. Temperature itself is the average kinetic energy of an object that is a measurement of thermal energy. Remember that temperature itself is an intensive property. It is the inside or innate properties of a substance that is independent on the amount of that substance. For example, if we have a cup of water that's 100°C versus a gallon of water that's 100°C. The amount of water is different, but the temperature is the same. Because the temperature is the waters are at at the moment. Doesn't matter how much of the water we have. OK, so temperature is an intensive property.
Heat, on the other hand, is not. Heat is the flow of thermal energy from an object at a higher temperature to an object at a lower temperature. So it's normal for heat to move from a hotter object to a colder object. If you have a hotter object next to a colder object, eventually all the heat from the hotter object moves towards the colder one. So to heat it up, Heat itself is based on the amount of the substance present, so it would represent an extensive property. Now knowing the difference between temperature and heat is critical.
For the example that's left right below it. If we take a look at this example question, it says from the image provided below, determine which part of the cubes represent temperature and which part represents heat. All right, so we have two cubes here. We can see that the cube on the left has those little red balls that seem to be bouncing everywhere. They're highly energetic. That happens because the overall temperature in this cube is higher. So we'd say that this cube has a higher temperature. Then if we look on the other side, we see that they are kind of like stuck in place. They're not moving at the same speed as before. That's because they are at a lower temperature.
And remember we just said that heat goes from a higher temperature to a lower temperature object. That is explained by these WAVY lines. So here we see that heat is leaving the left cube and going to the right cube. So these WAVY lines represent heat. So that's how you need to understand temperature is an actual measurement. 100° twenty degrees 98°F. These are actual numbers. Heat is just a flow of thermal energy from a hotter object to a colder object. Although they're used interchangeably, sometimes they are not the same thing.
Now that we've talked about the difference between intensive when it comes to temperature and extensive when it comes to heat, let's move on to our next practice question.