So at this point we have identified three ways to determine the enthalpy or heat of reaction, otherwise known as ΔHreaction. Here we have constant volume calorimetry which uses a bomb calorimeter to find the enthalpy of reaction or heat of reaction of combustion reactions. Then we have thermochemical equations which uses stoichiometry and a balanced equation to find ΔHreaction. Then finally Hess's law. If you haven't seen it yet, it uses the enthalpy of partial reactions to find ΔHreaction for the overall reaction.
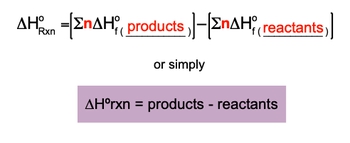
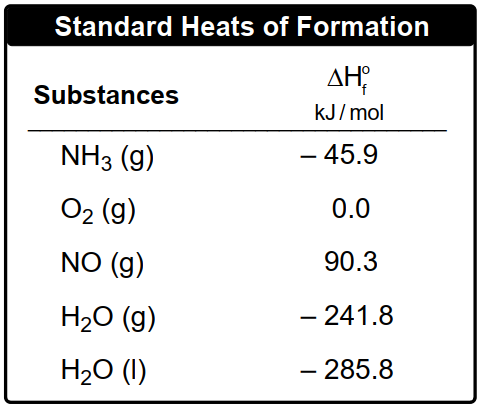
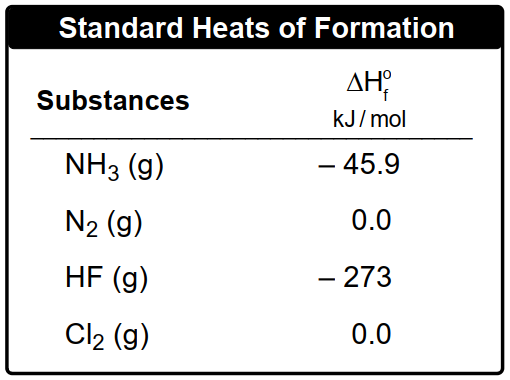
Now if the first three ways are unavailable, then we use the standard enthalpy of formation for substances to find the ΔHreaction we're going to say. Recall that an element in its standard state is given an enthalpy of formation of 0. So here we can use our standard heat of reaction formula to help us find ΔHreaction. Here it's ΔHreaction in its standard state. That's what this little 0 means. Here equals the summation for the entropies of formation of products minus the summation of the enthalpies of formation for reactants.
Or broken down in its simplest way, ΔHreaction equals products minus reactants O. Here ΔHreaction with a little circle on top. This is the standard enthalpy or reaction heat of reaction in kilojoules. This Sigma sign just means a summation or sum up and we'll see how that works. Here N equals the moles of your substance. And then here we're going to say that ΔHf with the little circle equals the standard enthalpy or heat of formation for substance in kilojoules over moles or sometimes joules over moles, depending on what units they are. It's always going to be kilojoules or joules over moles O.
Now that we've seen this standard heat of form of reaction formula, we're going to see how it works and use it in the next video. So click on the next video and let's take a look at an example question.