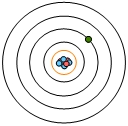
The first quantum number of interest is the principal quantum number. It uses the variable N and the principal quantum number deals with the size of the shell, and the size of a shell is equal to the energy level of that shell. What we need to realize here is the value of N increases, then both the size and energy level of an atomic orbital will also increase.
Now we're going to say here that the energy levels, or shell numbers of an atom can be tied to the periods or roles of the periodic table. So here we take a look at an atom. Here we have our nucleus in the middle. We have here our first shell, so N=1 all the way to our 7th shell here, which is N=7. These shell numbers are connected to the periods or rows of the periodic table. So shell one is connected to. One and as you can see we want all the way up to to shell 7. And looking at the periodic table, we have periods 1 to seven.
Now here's the thing, the periodic table, if you've watched my videos on it, we know that the periodic table is not a static thing, meaning that it's not going to stay in this form forever. In fact. 7, a lot of those elements in. Seven were only discovered within the past century. That's because as we explore new places on Earth, as we explore the universe, as our technology becomes more advanced, we'll discover new elements, we'll create new elements. So theoretically, the number of rows in the periodic table is infinite. Right now we have 7 rows, but it can increase to 8 rows, 10 rows in 1000 years.
So just realize here that the number of rows for the periodic table doesn't stop at 7, it's only 7. Now because of where we are technologically. In the future there's going to be more than 7 rows, so we're going to find atoms that go beyond seven. This ties into the limitation of the principal quantum number. We're going to say here that the principal quantum number N must be an integer, so a whole number. And since it's connected to the periods of the periodic table, it has to start off at one at the lowest as its lowest possible value.
And we just said that the number of rows is not static, it increases over time. So N theoretically can be any whole number from one to Infinity. So I just realized that on an exam or a quiz you see n=22. That's possible, we just haven't gotten there yet. So just remember the principle quantum number is connected to the size and energy of a shell and it affects the size and energy of the orbitals.