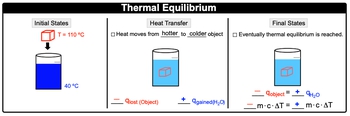
Thermal equilibrium is when 2 substances in physical contact of one another are at the same temperature. Now at the same temperature, these two substances would no longer exchange thermal energy by way of the first law of thermodynamics. So if we take a look here, let's say we have this heated metal cube here and it's initially at a temperature of 110�C and we're going to place it into water at 40�C.
Remember, heat transfers when it comes to heat. It always moves from hotter to colder object. So the cube will be losing some heat. So some of the heat from the cube will be lost and it's going to go into the water. So we'd say here that the cube is losing heat, so it would have a negative cube, and the water is absorbing that heat, so it would be a positive cue for the water. Eventually though, the queue will no longer be able to release any more heat because it'll get to the same temperature as the water. O it's at that moment we've reached thermal equilibrium.
Now, at thermal equilibrium, we can say here that they're both going to have the same heat, so the negative Q of the object hotter object would be equal to the positive Q of water in this case. And if their queues or heats are equal to one another, then their M cap formulas are equal to each other because remember Q equals MCAT, so negative MCAT equals positive MCAT. Now under ideal thermal equilibrium, heat transfers only occur between the solvent and the immersed heated object.
If the situation is not ideal then then we can say that that additional HE could be absorbed by the calorimeter. So the calorimeter or the container in this case same thing would absorb some of that heat of the object. O here this equation would expand out under non ideal conditions to be negative Q of the object equals positive Q of the water plus Q of the container also known as the calorimeter. So just remember if your rofessor is mentioning that this is a pure thermal equilibrium type of question, then you could just say Q negative Q hotter object equals positive Q of colder object. But if the calorimeter is involved, you have to take into account that it too would absorb some of this heat that's coming off of the hotter object.