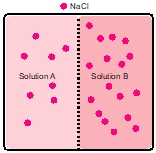
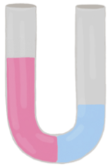
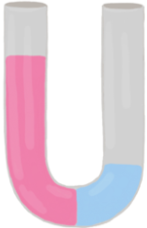
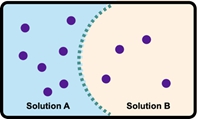
Osmosis refers to the net movement of a solvent, primarily water, through a semipermeable membrane. This type of membrane selectively allows certain substances to pass while blocking others, particularly larger molecules and solutes such as ions. In biological systems, cell membranes function as semipermeable barriers, enabling the passage of water while restricting the movement of solutes.
To illustrate this concept, consider a semipermeable membrane depicted as a gatekeeper. Larger molecules, represented in red, are unable to cross the membrane due to their size, while smaller molecules that meet specific criteria are permitted entry. This selective permeability is crucial for maintaining cellular homeostasis, as it regulates the internal environment of cells by controlling the influx and efflux of various substances.
In summary, osmosis is essential for the movement of water across cell membranes, which play a vital role in the overall function and health of living cells by preventing unwanted solutes from entering or exiting the cell.