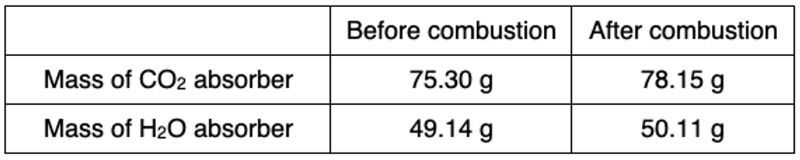
The compound that makes up an experimental jet contains carbon, hydrogen, and oxygen. Suppose that in one experiment, the combustion of 11.5g of the liquid gave the following results. Here we're given the masses of carbon dioxide and water before combustion and after combustion. Remember, when it comes to our combustion apparatus, we have our chambers, which basically collect the amount of carbon dioxide and water that's produced during a combustion reaction. Within these chambers, there had already been some carbon dioxide and some water, and what we're seeing is after combustion, how much more water and carbon dioxide was created.
From this information, we need to determine the empirical formula of the unknown liquid. Alright, so if we take a look at the steps, so step zero, we're going to subtract the grams after combustion by the grams before combustion to find the grams of CO2 and water. All right, so we're going to say we have 32.815 grams of CO2 after combustion, -10.815 grams of CO2 before combustion. That tells us how much CO2 was created. When we do that, we're going to get 22.0 grams of CO2 and then we're going to do 23.610 grams of water minus 10.110 grams of water. So that gives me 13.5 grams of water. So this is how much CO2 and water that was produced.
So at this point it then continues into a regular combustion analysis type of question. We're going to do step one, where we're going to convert the grams of CO2 into moles or into grams, actually grams of carbon. So we're going to have 22.0 grams of CO2. What we want to do first is we want to cancel out. It's right over here. We have 22 grams of CO2. We want to change those grams of CO2 into moles of CO2, so one mole of CO2 on top. At this point we've seen carbon dioxide numerous times, so the one carbon and the two oxygens weigh 44.01 grams. Grams cancel out. Now convert the moles of CO2 into just moles of carbon. Within this formula, we have only one carbon, so that's one mole of C and then finally for every one mole of C we're told according to the periodic table, its atomic mass is 12.01 grams. When we do that, we're going to get the mass of C, which comes out to 6.0036 grams of C.
Next we're going to convert grams of water into grams of hydrogen, so we have 13.5 grams of water. We're going to say here that one mole of water which possesses 2 hydrogens and one oxygen is 18.016 grams. Then we're going to convert moles of water into just moles of H. And remember, for every one mole of water, we see that there's two hydrogens within the formula. And then convert the one mole of H into grams of H. So when we do that, we're going to get 1.5107 grams of H.
Step three if necessary. Subtract the grams of step one and two from the grams of the sample to determine the third element. What we're told within the question that our third element that makes up this jet fuel is oxygen. Our total amount of the liquid, which contains carbon, hydrogen and oxygen, is 11.5, so we're going to do 11.5 grams, which contains all three elements. Subtract out the grams of carbon plus the grams of hydrogen gives us 3.9857 grams oxygen.
Now that we have the grams of all three elements that comprise my compound, we move on to step 4. In step 4, you convert all the masses into moles. We're going to bring down these masses here, so 6.0036 grams carbon, so one mole of C and on the bottom the mass of C. Then we have 1.5107 grams H. For every one mole H it's 1.008 grams H and then we have 3.9857 grams O, and so for every one mole of O, it's 16 grams. Remember, these masses are coming from the periodic table. When we do that, we're going to get the moles of each of these elements. This comes out to be 0.4999 moles of C. This equals 1.4987 moles of H and this equals 0.2491 moles of O.
At this point, step five, you divide each mole answer by the smallest mole value in order to obtain whole numbers for each element. So our smallest moles that we obtained were the moles of the oxygen. So everyone gets divided by 0.2491. So when we do that, that's going to give us ratios of each of these elements. So that gives me two carbons, 6 hydrogens and one oxygen. Now, since we got whole numbers, we don't have to worry about rounding, but if we did, we'd look at step 6. If you get a value of 0.1 or 0.9, then you can round. But again, at this point we've determined what our empirical formula is. We've determined that it's C2H6O in terms of our answer.